Summary.
We have previously shown that protease-resistant and highly immunoreactive compact NP oligomers, dissociating at +80 °C and possessing properties of folded proteins, are post-translationally formed in influenza-virus-infected cells. In this study we demonstrate that, in addition to compact NP oligomers, incompletely folded NP multimers are detected intracellularly by SDS/PAGE carried out under weak dissociating conditions. In cells infected with avian, human A(H2N2), and human A(H3N2) viruses, NP multimers are detected in the stacking gel of SDS/PAGE as retarded and loose structures dissociating at +50 °C. NP multimers are more sensitive to proteolysis than NP oligomers, but they are more resistant to proteolysis than NP monomers. In contrast to compact NP oligomers, NP multimers possess a weak immunoreactivity to some monoclonal antibodies. Pulse-chase experiments have shown that NP multimers appear at early stages of NP synthesis and are partially converted post-translationally into faster-migrating compact NP oligomers. In the course of infection, the excess NP multimers not converted into compact NP oligomers accumulate in cells and degrade.
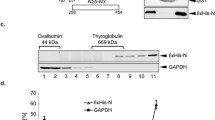
Under weak dissociating conditions, intracellular NP multimers are relatively stable in avian, human A(H2N2) and human A(H3N2) viruses and unstable in human A(H1N1) viruses, dissociating into monomers.
NP multimers presumably serve to bring nascent unfolded NP molecules into close contact with each other for further oligomerization, to protect NP monomers from proteolysis, and to serve as intermediates in the posttranslational folding of NP.
Similar content being viewed by others
References
AS de Jong WJ Melchers DH Glaudemans PH Willems FJ van Kuppeveld (2004) ArticleTitleMutational analysis of different regions in the coxsackievirus 2B protein: requirements for homo-multimerization, membrane permeabilization, subcellular localization, and virus replication J Boil Chem 279 19924–19935 10.1074/jbc.M314094200 Occurrence Handle10.1074/jbc.M314094200 Occurrence Handle1:CAS:528:DC%2BD2cXjs1Wiu7g%3D
A de Silva I Braakman A Helenius (1993) ArticleTitlePosttranslational folding of VSV G protein in the ER: involvement of noncovalent and covalent complexes J Cell Biol 120 647–655 10.1083/jcb.120.3.647 Occurrence Handle10.1083/jcb.120.3.647 Occurrence Handle1:CAS:528:DyaK3sXnslOnsg%3D%3D Occurrence Handle8381122
RW Doms A Ruusala C Machamer J Helenius A Helenius JK Rose (1988) ArticleTitleDifferential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of VSV G protein J Cell Biol 107 89–99 10.1083/jcb.107.1.89 Occurrence Handle10.1083/jcb.107.1.89 Occurrence Handle1:CAS:528:DyaL1cXkslSitro%3D Occurrence Handle2839523
D Elton E Medcalf K Bishop P Digard (1999) ArticleTitleOligomerization of the influenza virus nucleoprotein: identification of positive and negative sequence elements Virology 260 190–200 10.1006/viro.1999.9818 Occurrence Handle10.1006/viro.1999.9818 Occurrence Handle1:CAS:528:DyaK1MXktlynsbw%3D Occurrence Handle10405371
HC Hsieh TK Kumar CC Chiu C Yu (2005) ArticleTitleEquilibrium unfolding of an oligomeric protein involves formation of a multimeric intermediate state(s) Biochem Biophys Res Commun 326 108–114 10.1016/j.bbrc.2004.10.211 Occurrence Handle10.1016/j.bbrc.2004.10.211 Occurrence Handle1:CAS:528:DC%2BD2cXhtVagsrrN Occurrence Handle15567159
R Jaenicke (1991) ArticleTitleProtein folding: local structures, domains, subunits, and assemblies Biochemistry 30 3147–3161 10.1021/bi00227a001 Occurrence Handle10.1021/bi00227a001 Occurrence Handle1:CAS:528:DyaK3MXhtlyis7o%3D Occurrence Handle2009257
Y Kawaoka S Krauss RG Webster (1989) ArticleTitleAvian-to human transmission of PB1 gene of influenza A viruses in 1957 and 1968 pandemics J Virol 63 4603–4608 Occurrence Handle1:CAS:528:DyaK3cXntFWjtQ%3D%3D Occurrence Handle2795713
UK Laemmli (1970) ArticleTitleCleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature 227 680–685 10.1038/227680a0 Occurrence Handle10.1038/227680a0 Occurrence Handle1:CAS:528:DC%2BD3MXlsFags7s%3D Occurrence Handle5432063
G Leone MC Coffey R Gilmore R Duncan L Maybaum PW Lee (1996) ArticleTitleC-terminal trimerization, but not N-terminal trimerization, of the reovirus cell attachment protein is posttranslational and Hsp70/ATP-dependent process J Biol Chem 271 8466–8471 10.1074/jbc.271.14.8466 Occurrence Handle10.1074/jbc.271.14.8466 Occurrence Handle1:CAS:528:DyaK28XitFaqsro%3D Occurrence Handle8626547
J Martin-Benito E Area J Ortega O Liorca JM Valpuesta JI Carrascosa J Ortin (2001) ArticleTitleThree-dimensional reconstruction of a recombinant influenza virus ribonucleoprotein particle EMBO Rep 2 313–317 10.1093/embo-reports/kve063 Occurrence Handle10.1093/embo-reports/kve063 Occurrence Handle1:CAS:528:DC%2BD3MXks1Cqtbg%3D Occurrence Handle11306552
J Ortega J Martin-Benito T Zurcher JM Valpuesta JL Carrascosa J Ortin (2000) ArticleTitleUltrastructural and functional analyses of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification J Virol 74 156–163 10.1128/JVI.74.1.156-163.2000 Occurrence Handle10.1128/JVI.74.1.156-163.2000 Occurrence Handle1:CAS:528:DC%2BD3cXkvVCh Occurrence Handle10590102
A Portela P Digard (2002) ArticleTitleThe influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication J Gen Virol 83 723–734 Occurrence Handle1:CAS:528:DC%2BD38XislGhsrY%3D Occurrence Handle11907320
EN Prokudina-Kantorovich NP Semenova (1996) ArticleTitleIntracellular oligomersization of influenza virus nucleoprotein Virology 223 51–56 10.1006/viro.1996.0454 Occurrence Handle10.1006/viro.1996.0454 Occurrence Handle1:CAS:528:DyaK28XlsVemsbc%3D Occurrence Handle8806539
EN Prokudina NP Semenova VM Chumakov IA Rudneva (2004) ArticleTitleTransient disulfide bonds formation in conformational maturation of influenza virus Nucleocapsid protein Virus Res 2 169–175 10.1016/j.virusres.2003.11.008 Occurrence Handle10.1016/j.virusres.2003.11.008
EN Prokudina NP Semenova VM Chumakov (2005) ArticleTitleStability of intracellular influenza virus nucleocapsid protein oligomers Arch Virol 150 833–839 10.1007/s00705-004-0425-5 Occurrence Handle10.1007/s00705-004-0425-5 Occurrence Handle1:CAS:528:DC%2BD2MXisV2hsbg%3D Occurrence Handle15645379
RW Ruigrok F Baudin (1995) ArticleTitleStructure of influenza virus RNP particles. 11. Purified RNA-free influenza virus RNP forms structures that are indistinguishable from the intact influenza virus RNP particles J Gen Virol 76 1009–1014 10.1099/0022-1317-76-4-1009 Occurrence Handle10.1099/0022-1317-76-4-1009 Occurrence Handle1:CAS:528:DyaK2MXksl2nsrk%3D Occurrence Handle9049350
LM Selimova VM Zaides VM Zhdanov (1982) ArticleTitleDisulfide bonding in influenza virus proteins as revealed by polyacrilamide gel electrophoresis J Virol 44 450–457 Occurrence Handle1:CAS:528:DyaL38XmtFSrs7g%3D Occurrence Handle7143574
VN Uversky DJ Segel S Doniach AL Fink (1998) ArticleTitleAssociation-induced folding of globular proteins Proc Natl Acad Sci USA 95 5480–5483 10.1073/pnas.95.10.5480 Occurrence Handle10.1073/pnas.95.10.5480 Occurrence Handle1:CAS:528:DyaK1cXjtFWkt70%3D Occurrence Handle9576907
JM Yon (1997) ArticleTitleProtein folding: concepts and perspectives Cell Mol Life Sci 53 557–567 10.1007/s000180050072 Occurrence Handle10.1007/s000180050072 Occurrence Handle1:CAS:528:DyaK2sXltFyjsbo%3D Occurrence Handle9312402
H Zhao M Ekstrom H Garoff (1998) ArticleTitleThe M1 and NP proteins of influenza A virus form homo- but not heterooligomeric complexes when coexpressed in BHK-21 cells J Gen Virol 79 2435–2446 Occurrence Handle1:CAS:528:DyaK1cXmsVGnsL4%3D Occurrence Handle9780049
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prokudina, E., Semenova, N., Chumakov, V. et al. Non-compact nucleocapsid protein multimers in influenza-virus-infected cells. Arch Virol 152, 981–988 (2007). https://doi.org/10.1007/s00705-006-0911-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-006-0911-z