Summary.
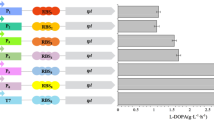
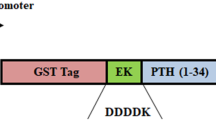
Wild-type and N-terminal 35-, 38-, and 44-amino acid-deleted mutants of human tyrosine hydroxylase type 1 (hTH1) fused to maltose-binding protein via the target sequence for a restriction protease were expressed in Escherichia coli and purified. The fused protein was treated with the restriction protease factor Xa or enterokinase to isolate hTH1 from the fused form. The treatment of fused wild-type and 35-amino acid-deleted mutant with factor Xa and enterokinase caused non-specific cleavages in the vicinity of the phosphorylation sites, Ser19 and Ser40, due to the flexible conformation of the N-terminus of hTH1.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received April 27, 1999; accepted June 16, 1999
Rights and permissions
About this article
Cite this article
Nakashima, A., Mori, K., Nagatsu, T. et al. Expression of human tyrosine hydroxylase type I in Escherichia coli as a protease-cleavable fusion protein. J Neural Transm 106, 819–824 (1999). https://doi.org/10.1007/s007020050202
Issue Date:
DOI: https://doi.org/10.1007/s007020050202