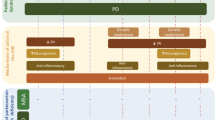
Abstract
Emerging studies suggest a correlation between elevated plasma homocysteine (hcy) levels and the risk of atherosclerosis, vascular disorders, and neurodegenerative diseases, including Parkinson's disease (PD). This narrative review delves into the intricate relationships between Hcy, vitamin B metabolites, dopamine-substituting compounds, and various symptoms of PD. Patients undergoing a long-term L-dopa/dopa-decarboxylase inhibitor (DDI) regimen, especially without a concurrent catechol-O-methyl transferase (COMT) inhibitor or methyl group-donating vitamin supplementation, such as vitamins B6 and B12, exhibit an elevation in Hcy and a decline in vitamin B metabolites. These altered concentrations appear to be associated with heightened risks of developing non-motor symptoms, including peripheral neuropathy and cognitive disturbances. The review underscores the impact of levodopa metabolism via COMT on homocysteine levels. In light of these findings, we advocate for the supplementation of methyl group-donating vitamins, notably B6 and B12, in patients undergoing a high-dose L-dopa/DDI regimen, particularly those treated with L-dopa/carbidopa intestinal gel (LCIG) infusion.

Similar content being viewed by others
Data availability
Data supporting the findings of this study is available from the corresponding author, [Bhidayasiri R], upon reasonable request.
References
Aden E, Carlsson M, Poortvliet E, Stenlund H, Linder J, Edstrom M, Forsgren L, Haglin L (2011) Dietary intake and olfactory function in patients with newly diagnosed Parkinson’s disease: a case-control study. Nutr Neurosci 14:25–31
Anamnart C, Kitjarak R (2021) Effects of vitamin B12, folate, and entacapone on homocysteine levels in levodopa-treated Parkinson’s disease patients: a randomized controlled study. J Clin Neurosci 88:226–231
Antonini A, Bondiolotti G, Natuzzi F, Bareggi SR (2010) Levodopa and 3-OMD levels in Parkinson patients treated with Duodopa. Eur Neuropsychopharmacol 20:683–687
Baviera-Munoz R, Buigues-Lafuente A, Campins-Romeu M, Garces-Sanchez M, Martinez-Torres I (2022) Refractory status epilepticus due to vitamin B(6) deficit in a Parkinson’s disease patient in treatment with levodopa/carbidopa intestinal gel. Neurologia (engl Ed) 37:608–609
Belcastro V, Pierguidi L, Castrioto A, Menichetti C, Gorgone G, Ientile R, Pisani F, Rossi A, Calabresi P, Tambasco N (2010) Hyperhomocysteinemia recurrence in levodopa-treated Parkinson’s disease patients. Eur J Neurol 17:661–665
Ben Shlomo Y, Marmot MG (1995) Survival and cause of death in a cohort of patients with parkinsonism: Possible clues to aetiology? J Neurol Neurosurg Psychiatry 58:293–299
Bialecka M, Robowski P, Honczarenko K, Roszmann A, Slawek J (2009) Genetic and environmental factors for hyperhomocysteinaemia and its clinical implications in Parkinson’s disease. Neurol Neurochir Pol 43:272–285
Boelens Keun JT, Arnoldussen IA, Vriend C, Van De RO (2021) Dietary approaches to improve efficacy and control side effects of levodopa therapy in Parkinson’s disease: a systematic review. Adv Nutr 12:2265–2287
Botez MI, Peyronnard JM, Bachevalier J, Charron L (1978) Polyneuropathy and folate deficiency. Arch Neurol 35:581–584
Bottiglieri T, Hyland K, Reynolds EH (1994) The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs 48(2):137–152. https://doi.org/10.2165/00003495-199448020-00002
Brosnan JT, Jacobs RL, Stead LM, Brosnan ME (2004) Methylation demand: a key determinant of homocysteine metabolism. Acta Biochim Pol 51:405–413
Ceravolo R, Cossu G, Bandettini di Poggio M, Santoro L, Barone P, Zibetti M, Frosini D, Nicoletti V, Manganelli F, Iodice R, Picillo M, Merola A, Lopiano L, Paribello A, Manca D, Melis M, Marchese R, Borelli P, Mereu A, Contu P, Abbruzzese G, Bonuccelli U (2013) Neuropathy and levodopa in Parkinson’s disease: evidence from a multicenter study. Mov Disord 28(10):1391–1397. https://doi.org/10.1002/mds.25585
Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Logroscino G, Willett WC, Ascherio A (2004) Folate intake and risk of Parkinson’s disease. Am J Epidemiol 160:368–375
Choi YJ, Choi IY, Jang W, Jeong SM, Park S, Han K, Lee Y, Lee DH, Shin DW (2021) Gastrectomy, vitamin B12 supplementation and the risk of Parkinson’s disease: a nationwide cohort study. Parkinsonism Relat Disord 83:15–21
Christine CW, Auinger P, Joslin A, Yelpaala Y, Green R (2018) Vitamin B12 and homocysteine levels predict different outcomes in early Parkinson’s disease. Mov Disord 33:762–770
Christine CW, Auinger P, Saleh N, Tian M, Bottiglieri T, Arning E, Tran NK, Ueland PM, Green R (2020) Relationship of cerebrospinal fluid vitamin B12 status markers with Parkinson’s disease progression. Mov Disord 35:1466–1471
de Lau LM, Koudstaal PJ, Witteman JC, Hofman A, Breteler MM (2006) Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology 67:315–318
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Fan X, Zhang L, Li H, Chen G, Qi G, Ma X, Jin Y (2020) Role of homocysteine in the development and progression of Parkinson’s disease. Ann Clin Transl Neurol 7(11):2332–2338. https://doi.org/10.1002/acn3.51227
Goel A, Narayan SK, Sugumaran R (2022) Neuropsychiatric features, health-related quality of life, and caregiver burden in Parkinson’s disease. Ann Indian Acad Neurol 25:1147–1152
Haglin L, Johansson I, Forsgren L, Backman L (2017) Intake of vitamin B before onset of Parkinson’s disease and atypical parkinsonism and olfactory function at the time of diagnosis. Eur J Clin Nutr 71:97–102
Hassin-Baer S, Cohen O, Vakil E, Sela BA, Nitsan Z, Schwartz R, Chapman J, Tanne D (2006) Plasma homocysteine levels and Parkinson disease: disease progression, carotid intima-media thickness and neuropsychiatric complications. Clin Neuropharmacol 29:305–311
Isobe C, Abe T, Terayama Y (2010) L-Dopa therapy increases homocysteine concentration in cerebrospinal fluid from patients with Parkinson’s disease. J Clin Neurosci 17:717–721
Jugel C, Ehlen F, Taskin B, Marzinzik F, Muller T, Klostermann F (2013) Neuropathy in Parkinson’s disease patients with intestinal levodopa infusion versus oral drugs. PLoS ONE 8:e66639
Klostermann F, Jugel C, Muller T, Marzinzik F (2012) Malnutritional neuropathy under intestinal levodopa infusion. J Neural Transm 119:369–372
Kocer B, Guven H, Comoglu SS (2016) Homocysteine levels in Parkinson’s disease: Is entacapone effective? Biomed Res Int 2016:7563705
Kuhn W, Roebroek R, Blom H, Van OD, Przuntek H, Kretschmer A, Buttner T, Woitalla D, Muller T (1998) Elevated plasma levels of homocysteine in Parkinson’s disease. Eur Neurol 40:225–227
Lamberti P, Zoccolella S, Iliceto G, Armenise E, Fraddosio A, De MM, Livrea P (2005) Effects of levodopa and COMT inhibitors on plasma homocysteine in Parkinson’s disease patients. Mov Disord 20:69–72
Lee SH, Kim MJ, Kim BJ, Kim SR, Chun S, Kim HK, Ryu JS, Kim GS, Lee MC, Chung SJ, Koh JM (2010) Hyperhomocysteinemia due to levodopa treatment as a risk factor for osteoporosis in patients with Parkinson’s disease. Calcif Tissue Int 86:132–141
Licking N, Murchison C, Cholerton B, Zabetian CP, Hu SC, Montine TJ, Peterson-Hiller AL, Chung KA, Edwards K, Leverenz JB, Quinn JF (2017) Homocysteine and cognitive function in Parkinson’s disease. Parkinsonism Relat Disord 44:1–5
Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-d-aspartate receptor. Proc Natl Acad Sci U S A 94(11):5923–5928. https://doi.org/10.1073/pnas.94.11.5923
Lizarraga KJ, Lang AE (2022) Vitamins B6 and B12, levodopa, and their complex interactions in patients with Parkinson’s disease. Brain 145:e77–e78
Loens S, Chorbadzhieva E, Kleimann A, Dressler D, Schrader C (2017) Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behav 7:e00698
Martignoni E, Tassorelli C, Nappi G, Zangaglia R, Pacchetti C, Blandini F (2007) Homocysteine and Parkinson’s disease: A dangerous liaison? J Neurol Sci 257:31–37
Martinez-Martin P, Skorvanek M, Henriksen T, Lindvall S, Domingos J, Alobaidi A, Kandukuri PL, Chaudhari VS, Patel AB, Parra JC, Pike J, Antonini A (2023) Impact of advanced Parkinson’s disease on caregivers: an international real-world study. J Neurol 270:2162–2173
McCarter SJ, Stang C, Turcano P, Mielke MM, Ali F, Bower JH, Savica R (2020) Higher vitamin B12 level at Parkinson’s disease diagnosis is associated with lower risk of future dementia. Parkinsonism Relat Disord 73:19–22
McCully KS (2015) Homocysteine metabolism, atherosclerosis, and diseases of aging. Compr Physiol 6(1):471–505. https://doi.org/10.1002/cphy.c150021
Merola A, Romagnolo A, Zibetti M, Bernardini A, Cocito D, Lopiano L (2016) Peripheral neuropathy associated with levodopa–carbidopa intestinal infusion: a long-term prospective assessment. Eur J Neurol 23:501–509
Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ (2002) Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology 58:1471–1475
Mudd SH, Ebert MH, Scriver CR (1980) Labile methyl group balances in the human: the role of sarcosine. Metabolism 29(8):707–720. https://doi.org/10.1016/0026-0495(80)90192-4
Muller T (2008) Role of homocysteine in the treatment of Parkinson’s disease. Expert Rev Neurother 8:957–967
Muller T (2011) Motor complications, levodopa metabolism and progression of Parkinson’s disease. Expert Opin Drug Metab Toxicol 7:847–855
Muller T (2022) What are the main considerations when prescribing pharmacotherapy for Parkinson’s disease? Expert Opin Pharmacother 23:745–750
Muller T, Kuhn W (2006) Tolcapone decreases plasma levels of S-adenosyl-l-homocysteine and homocysteine in treated Parkinson’s disease patients. Eur J Clin Pharmacol 62:447–450
Muller T, Kuhn W (2009) Homocysteine levels after acute levodopa intake in patients with Parkinson’s disease. Mov Disord 24:1339–1343
Muller T, Muhlack S (2009) Peripheral COMT inhibition prevents levodopa associated homocysteine increase. J Neural Transm 116:1253–1256
Muller T, Werne B, Fowler B, Kuhn W (1999) Nigral endothelial dysfunction, homocysteine, and Parkinson’s disease. Lancet 354:126–127
Muller T, Woitalla D, Fowler B, Kuhn W (2002) 3-OMD and homocysteine plasma levels in parkinsonian patients. J Neural Transm 109:175–179
Muller T, Renger K, Kuhn W (2004) Levodopa-associated increase of homocysteine levels and sural axonal neurodegeneration. Arch Neurol 61:657–660
Muller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Goetze O, Woitalla D (2006) Pharmacokinetic behaviour of levodopa and 3-O-methyldopa after repeat administration of levodopa/carbidopa with and without entacapone in patients with Parkinson’s disease. J Neural Transm 113:1441–1448
Muller T, Jugel C, Ehret R, Ebersbach G, Bengel G, Muhlack S, Klostermann F (2011a) Elevation of total homocysteine levels in patients with Parkinson’s disease treated with duodenal levodopa/carbidopa gel. J Neural Transm 118:1329–1333
Muller T, Jugel C, Ehret R, Ebersbach G, Bengel G, Muhlack S, Klostermann F (2011b) Elevation of total homocysteine levels in patients with Parkinson’s disease treated with duodenal levodopa/carbidopa gel. J Neural Transm (vienna) 118:1329–1333
Muller T, Woitalla D, Muhlack S (2011c) Inhibition of catechol-O-methyltransferase modifies acute homocysteine rise during repeated levodopa application in patients with Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol 383:627–633
Muller T, Van LT, Cornblath DR, Odin P, Klostermann F, Grandas FJ, Ebersbach G, Urban PP, Valldeoriola F, Antonini A (2013) Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord 19:501–507
Muller T, Jugel C, Muhlack S, Klostermann F (2013) Methyl group-donating vitamins elevate 3-O-methyldopa in patients with Parkinson disease. Clin Neuropharmacol 36:52–54
Muller T, Schlegel E, Zingler S, Thiede HM (2022) Effects of one-day application of levodopa/carbidopa/entacapone versus levodopa/carbidopa/opicapone in Parkinson’s disease patients. Cells 11:1
Nakaso K, Yasui K, Kowa H, Kusumi M, Ueda K, Yoshimoto Y, Takeshima T, Sasaki K, Nakashima K (2003) Hypertrophy of IMC of carotid artery in Parkinson’s disease is associated with L-DOPA, homocysteine, and MTHFR genotype. J Neurol Sci 207:19–23
Nevrly M, Kanovsky P, Vranova H, Langova K, Hlustik P (2009) Effect of levodopa and entacapone treatment on plasma homocysteine levels in Parkinson’s disease patients. Parkinsonism Relat Disord 15:477–478
Olanow CW, Obeso JA (2000) Pulsatile stimulation of dopamine receptors and levodopa-induced motor complications in Parkinson’s disease: implications for the early use of COMT inhibitors. Neurology 55:S72–S77
Ostrem JL, Kang GA, Subramanian I, Guarnieri M, Hubble J, Rabinowicz AL, Bronstein J (2005) The effect of entacapone on homocysteine levels in Parkinson disease. Neurology 64:1482
O’Suilleabhain PE, Bottiglieri T, Dewey RB Jr, Sharma S, Az-Arrastia R (2004) Modest increase in plasma homocysteine follows levodopa initiation in Parkinson’s disease. Mov Disord 19:1403–1408
O’Suilleabhain PE, Oberle R, Bartis C, Dewey RB Jr, Bottiglieri T, Az-Arrastia R (2006) Clinical course in Parkinson’s disease with elevated homocysteine. Parkinsonism Relat Disord 12:103–107
Ozer F, Meral H, Hanoglu L, Aydemir T, Yilsen M, Cetin S, Ozturk O, Seval H, Koldas M (2006) Plasma homocysteine levels in patients treated with levodopa: motor and cognitive associations. Neurol Res 28:853–858
Ozkan S, Colak O, Kutlu C, Ertan M, Alatas O (2004) Plasma homocysteine levels in pergolide-treated Parkinson disease patients. Clin Neuropharmacol 27:163–165
Pauls KAM, Toppila J, Koivu M, Eerola-Rautio J, Udd M, Pekkonen E (2021) Polyneuropathy monitoring in Parkinson’s disease patients treated with levodopa/carbidopa intestinal gel. Brain Behav 11:e2408
Postuma RB, Espay AJ, Zadikoff C, Suchowersky O, Martin WR, Lafontaine AL, Ranawaya R, Camicioli R, Lang AE (2006) Vitamins and entacapone in levodopa-induced hyperhomocysteinemia: a randomized controlled study. Neurology 66:1941–1943
Przuntek H, Muller T, Riederer P (2004) Diagnostic staging of Parkinson’s disease: conceptual aspects. J Neural Transm 111:201–216
Rajabally YA, Martey J (2013) Levodopa, vitamins, ageing and the neuropathy of Parkinson’s disease. J Neurol 260:2844–2848
Ramachandran A, Jose J, Gafoor VA, Das S, Balaram N (2022) Prevalence and risk factors of peripheral neuropathy in Parkinson’s disease. Ann Indian Acad Neurol 25:1109–1115
Raman G, Tatsioni A, Chung M, Rosenberg IH, Lau J, Lichtenstein AH, Balk EM (2007) Heterogeneity and lack of good quality studies limit association between folate, vitamins B-6 and B-12, and cognitive function. J Nutr 137:1789–1794
Rispoli V, Simioni V, Capone JG, Golfre AN, Preda F, Sette E, Tugnoli V, Sensi M (2017) Peripheral neuropathy in 30 duodopa patients with vitamins B supplementation. Acta Neurol Scand 136:660–667
Romagnolo A, Merola A, Artusi CA, Rizzone MG, Zibetti M, Lopiano L (2019) Levodopa-induced neuropathy: a systematic review. Mov Disord Clin Pract 6(2):96–103. https://doi.org/10.1002/mdc3.12688
Sahu P, Thippeswamy H, Chaturvedi SK (2022) Neuropsychiatric manifestations in vitamin B12 deficiency. Vitam Horm 119:457–470
Sampedro F, Martinez-Horta S, Horta-Barba A, Grothe MJ, Labrador-Espinosa MA, Jesus S, Rmes-Gomez A, Carrillo F, Puig-Davi A, Lora FR, Barbera MA, Pastor P, Arroyo SE, Vila BS, Foraster AC, Martinez JR, Padilla FC, Morlans MP, Aramburu IG, Ceberio JI, Vara JH, de Fabregues-Boixar O, de Deus FT, Avila A, Martinez-Castrillo JC, Bejr-Kasem H, Campolongo A, Pascual-Sedano B, Martinez-Martin P, Santos-Garcia D, Mir P, Kulisevsky J (2022) Increased homocysteine levels correlate with cortical structural damage in Parkinson’s disease. J Neurol Sci 434:120148
Santos GD, Martinez Castrillo JC, Puente PV, Seoane UA, Fernandez DS, Benita LV, Udaeta BB, Campolongo PA, Mariscal PN (2016) Clinical management of patients with advanced Parkinson’s disease treated with continuous intestinal infusion of levodopa/carbidopa. Neurodegener Dis Manag 6:187–202
Santos-Garcia D, de Deus FT, Cores BC, Iniguez Alvarado MC, Feal Panceiras MJ, Suarez CE, Canfield H, Martinez MC, Jesus S, Aguilar M, Pastor P, Planellas L, Cosgaya M, Garcia CJ, Caballol N, Legarda I, Hernandez VJ, Cabo I, Lopez ML, Gonzalez AI, Vila Rivera MA, Gomez MV, Nogueira V, Puente V, Dotor Garcia-Soto J, Borrue C, Solano VB, Alvarez SM, Vela L, Escalante S, Cubo E, Carrillo PF, Martinez Castrillo JC, Sanchez AP, Onso Losada MG, Ariztegui NL, Gaston I, Kulisevsky J, Blazquez EM, Seijo M, Martinez JR, Valero C, Kurtis M, De FO, Gonzalez AJ, Alonso RR, Ordas C, Lopez DiazL LM, McAfee D, Martinez-Martin P, Mir P (2022) Predictors of the change in burden, strain, mood, and quality of life among caregivers of Parkinson’s disease patients. Int J Geriatr Psychiatry 37:1
Schroecksnadel K, Leblhuber F, Fuchs D (2004) Effect of L-dopa on plasma homocysteine in PD patients: relationship to B-vitamin status. Neurology 62:676–677
Shen L (2015) Associations between B vitamins and Parkinson’s disease. Nutrients 7:7197–7208
Silla Y, Varshney S, Ray A, Basak T, Zinellu A, Sabareesh V, Carru C, Sengupta S (2019) Hydrolysis of homocysteine thiolactone results in the formation of protein-cys-S-S-homocysteinylation. Proteins 87(8):625–634. https://doi.org/10.1002/prot.25681
Skodda S, Muller T (2013) Refractory epileptic seizures due to vitamin B6 deficiency in a patient with Parkinson’s disease under duodopa(R) therapy. J Neural Transm (vienna) 120(2):315–318. https://doi.org/10.1007/s00702-012-0856-1
Skovierova H, Vidomanova E, Mahmood S, Sopkova J, Drgova A, Cervenova T, Halasova E, Lehotsky J (2016) The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. https://doi.org/10.3390/ijms17101733
Song IU, Kim JS, Park IS, Kim YD, Cho HJ, Chung SW, Lee KS (2013) Clinical significance of homocysteine (hcy) on dementia in Parkinson’s disease (PD). Arch Gerontol Geriatr 57:288–291
Taher J, Naranian T, Poon YY, Merola A, Mestre T, Suchowersky O, Kulasingam V, Fasano A (2022) Vitamins and infusion of levodopa–carbidopa intestinal gel. Can J Neurol Sci 49:19–28
Takeuchi H, Kawashima R (2022) A prospective study on the relationship between iron supplement intake, hemoglobin concentration, and risk of Parkinsonism. Nutrients 14:1
Tchantchou F (2006) Homocysteine metabolism and various consequences of folate deficiency. J Alzheimers Dis 9:421–427
Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D (2008) Neuropathy as a potential complication of levodopa use in Parkinson’s disease. Mov Disord 23(13):1850–1859. https://doi.org/10.1002/mds.22137
Toth C, Breithaupt K, Ge S, Duan Y, Terris JM, Thiessen A, Wiebe S, Zochodne DW, Suchowersky O (2010) Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol 68:28–36
Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH (1993) Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem 39(9):1764–1779
Ulrey CL, Liu L, Andrews LG, Tollefsbol TO (2005) The impact of metabolism on DNA methylation. Hum Mol Genet 14 Spec No 1: R139-R147
Valkovic P, Benetin J, Blazicek P, Valkovicova L, Gmitterova K, Kukumberg P (2005) Reduced plasma homocysteine levels in levodopa/entacapone treated Parkinson patients. Parkinsonism Relat Disord 11:253–256
Vikdahl M, Domellof ME, Forsgren L, Haglin L (2015) Olfactory function, eating ability, and visceral obesity associated with MMSE three years after Parkinson’s disease diagnosis. J Nutr Health Aging 19:894–900
Weintraub D, Irwin D (2022) Diagnosis and treatment of cognitive and neuropsychiatric symptoms in Parkinson disease and dementia with lewy bodies. Continuum (minneap Minn) 28:1314–1332
Zesiewicz TA, Wecker L, Sullivan KL, Merlin LR, Hauser RA (2006) The controversy concerning plasma homocysteine in Parkinson disease patients treated with levodopa alone or with entacapone: effects of vitamin status. Clin Neuropharmacol 29:106–111
Zhang L, Yan J, Xu Y, Long L, Zhu C, Chen X, Jiang Y, Yang L, Bian L, Wang Q (2011) The combination of homocysteine and C-reactive protein predicts the outcomes of Chinese patients with Parkinson’s disease and vascular parkinsonism. PLoS ONE 6:e19333
Zhong M, Zhu S, Gu R, Wang Y, Jiang Y, Bai Y, Jiang X, Shen B, Yan J, Pan Y, Zhu J, Zhang L (2022) Elevation of plasma homocysteine and minor hallucinations in Parkinson’s disease: a cross-sectional study. Behav Neurol 2022:4797861
Zoccolella S, Lamberti P, Armenise E, De MM, Lamberti SV, Mastronardi R, Fraddosio A, Iliceto G, Livrea P (2005a) Plasma homocysteine levels in Parkinson’s disease: role of antiparkinsonian medications. Parkinsonism Relat Disord 11:131–133
Zoccolella S, Lamberti P, Iliceto G, Diroma C, Armenise E, Defazio G, Lamberti SV, Fraddosio A, De MM, Livrea P (2005b) Plasma homocysteine levels in L-dopa-treated Parkinson’s disease patients with cognitive dysfunctions. Clin Chem Lab Med 43:1107–1110
Acknowledgements
No funding are provided for this work.
Author information
Authors and Affiliations
Contributions
OP: conception for the article, performing literature search and data analysis, and writing of the first draft manuscript. RB: conception and organisation for the article, performing literature search and data analysis, and critique and writing of the revised draft manuscript. PG-R: conception and organisation for the article, review, and critique of the manuscript. WJ: conception and organisation for the article, review, and critique the manuscript. PO: conception and organisation for the article, review, and critique the manuscript. PR: conception and organisation for the article, review, and critique the manuscript. TM: conception and organisation for the article, review, and critique and writing of the revised draft manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest to declare.
Consent to participate
This study does not be required consent to participate due to its nature of being a review article.
Consent to publish
This study does not require consent to publish due to its nature of being review article.
Data and/or code availability
Not applicable.
Ethics approval
This study does not be required traditional ethical approval or follow the ethical guidelines due to its nature of being a review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phokaewvarangkul, O., Bhidayasiri, R., Garcia-Ruiz, P. et al. Homocysteine, vitamin B metabolites, dopamine-substituting compounds, and symptomatology in Parkinson’s disease: clinical and therapeutic considerations. J Neural Transm 130, 1451–1462 (2023). https://doi.org/10.1007/s00702-023-02684-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02684-9