Abstract
The cholinergic theory of depression highlights the involvement of muscarinic acetylcholine receptors in the neurobiology of mood disorders. The present study was designed to investigate the effect of sildenafil, a phosphodiesterase type 5 inhibitor which exhibits cholinomimetic properties, alone and in combination with scopolamine in the forced swim test in mice. Moreover, we assessed the ability of sildenafil to modify the antidepressant activity of two tricyclic antidepressants with distinct cholinolytic activity, amitriptyline and desipramine. Swim sessions were conducted by placing mice in glass cylinders filled with water for 6 min and the duration of behavioral immobility during the last 4 min of the test was evaluated. Locomotor activity was measured with photoresistor actimeters. To evaluate the potential pharmacokinetic interaction between amitriptyline and sildenafil, brain and serum concentrations of amitriptyline were determined by HPLC. Sildenafil (1.25–20 mg/kg) as well as scopolamine (0.5 mg/kg) and its combination with sildenafil (1.25 mg/kg) did not affect the total immobility time duration. However, joint administration of scopolamine with sildenafil at doses of 2.5 and 5 mg/kg significantly reduced immobility time as compared to control group. Moreover, co-administration of scopolamine with sildenafil at the highest dose (5 mg/kg) significantly decreased immobility time as compared to scopolamine-treated group. Sildenafil (1.25, 2.5 and 5 mg/kg) significantly enhanced the antidepressant activity of amitriptyline (5 mg/kg). No changes in anti-immobility action of desipramine (20 mg/kg) in combination with sildenafil (5, 10 and 20 mg/kg) were observed. Sildenafil did not affect amitriptyline level in both brain and serum. In conclusion, the present study suggests that sildenafil may enhance the activity of antidepressant drugs which exhibit cholinolytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depressive disorder is one of the most prevalent and disabling mental disorders which affects up to 20% of individuals at some point of their life (Pacher and Kecskemeti 2004). By 2020, depression is expected to become the second leading contributor to the global burden of disease worldwide (Murray and Lopez 1997). Depressed mood, loss of interest, feelings of worthlessness or inappropriate guilt, decrease in appetite and libido, insomnia and recurrent thoughts of death or suicide are the most common symptoms of depression (Maletic et al. 2007; Krishnan and Nestler 2008). Despite the widespread nature of depression, little is known about its etiology and pathophysiology. Interactions between genetic predispositions and environmental risk factors are thought to contribute to mood disorders (Jabbi et al. 2008). Due to complexity and heterogeneous character of depression, several distinct concepts regarding neurobiology of mood disorders have emerged. Among them, the monoamine theory of depression has gained the largest popularity (Nestler et al. 2002; Ansorge et al. 2007). This hypothesis proposes that depressive disorders are caused by deficits in serotonergic, noradrenergic and dopaminergic neurotransmitters systems. Although many experimental and clinical studies clearly implicated the involvement of monoamines in pathophysiology of depressive disorders, the monoaminergic hypothesis does not provide sufficient explanation for mechanism underlying depression and mode of action of antidepressant drugs (Manji et al. 2001; Krishnan and Nestler 2008).
Several lines of evidence indicate that hyperfunction of cholinergic neurotransmission contributes to depression (Pacher and Kecskemeti 2004; Mineur and Picciotto 2010). The so-called cholinergic–adrenergic theory of affective disorders was proposed for the first time almost 40 years ago by Janowsky et al. (1972). They assumed that the affective state is a result of balance between cholinergic and adrenergic neurotransmission in specific brain regions and that depression is associated with hyperactivity of cholinergic system while mania is a disease of hypocholinergic states (Janowsky et al. 1972, 1974). This hypothesis is consistent with observations that arecoline (a cholinomimetic) and physostigmine (an acetylcholinesterase inhibitor), presumably via muscarinic acetylcholine receptors, induce depression-like symptoms such as depressed mood, dysphoria, anhedonia and behavioral withdrawal in healthy volunteers (Janowsky et al. 1974; Dilsaver 1986; Mearns et al. 1994; Mineur and Picciotto 2010). On the basis of the theory that altered cholinergic neurotransmission leads to depression, the selective breeding program resulted in introduction of a strain of rats, called the Flinders Sensitive Line (FSL) rats, which are characterized by increased sensitivity to diisopropyl fluorophosphate, an acetylcholinesterase inhibitor. The FSL rats also exhibit certain behavioral symptoms of depression including reduced psychomotor activity and appetite (Overstreet and Russell 1982; Overstreet et al. 2005). Moreover, increased cholinergic activity was observed in rats subjected to swim stress (Dilsaver et al. 1986) and human post mortem studies revealed increased muscarinic receptors level in the brains of suicide victims (Meyerson et al. 1982). It has also been suggested that muscarinic receptors within the nucleus accumbens play a key role in the control of immobility state in the forced swim test in rodents (Chau et al. 1999, 2001). Further support for the idea that hyperfunction of muscarinic receptors may be involved in pathophysiology of depression provides results of clinical trials. The studies showed rapid and potent antidepressant response to antimuscarinic agent, scopolamine, in patients suffering from major depressive disorder and bipolar disorder (Furey and Drevets 2006; Drevets and Furey 2010).
Sildenafil, an active compound of Viagra®, is the first-line oral treatment for erectile dysfunctions of multiple etiologies (Rosen and McKenna 2002; Nurnberg and Hensley 2003). Mechanism of action of sildenafil involves the nitric oxide/cyclic guanosine 3′,5′-monophosphate/phosphodiesterase type 5 (NO/cGMP/PDE5) cell signaling pathway. Sildenafil works as a selective inhibitor of PDE5. By inhibiting cGMP degradation, it improves the relaxation of the smooth muscles in the corpus cavernosum and leads to erection (Ghofrani et al. 2006). Sildenafil exerts various effects on the central nervous system. The recent data show that it possesses neuroprotective properties, enhances neurogenesis and improves memory (Uthayathas et al. 2007). Furthermore, it has been showed that sildenafil, administered chronically, elevates muscarinic acetylcholine receptor signaling capacity in rats and exerts antidepressant-like properties in the forced swim test, a widely used animal model for screening antidepressants, after pre-treatment with atropine, a muscarinic receptor antagonist (Brink et al. 2008; Liebenberg et al. 2010a, b).
Therefore, the aim of the present study was to investigate the effect of sildenafil administered acutely on the animal behavior in the forced swim test in mice and to evaluate the anti-immobility action of sildenafil after central muscarinic receptor blockade with scopolamine. Since sildenafil possesses antidepressant properties which are attenuated because of its simultaneous cholinotropic effect (Brink et al. 2008; Liebenberg et al. 2010a, b), we presumed that it would potentiate the activity of amitriptyline, a commonly used antidepressant drug which exhibits cholinolytic properties (Snyder and Yamamura 1977; McKinney et al. 1988), and that it should not affect the activity of antidepressants devoid of antimuscarinic action, such as desipramine (Nelson 2009). To evaluate the potential pharmacokinetic interaction between amitriptyline and sildenafil, total brain and serum concentrations of amitriptyline were determined.
Materials and methods
Animals
Experimentally naïve male Albino Swiss mice (Laboratory Animals Breeding, Słaboszów, Poland) weighing 25–30 g were used in all experiments. The animals were housed in groups of 7–8 in polycarbonate cages at a controlled temperature (23–25°C), and humidity (50–60%) with 12 h light/dark cycle (lights on at 6:00 h). Tap water and food pellets (Agropol S.J., Motycz, Poland) were available ad libitum. All experiments were performed after at least 7 days of acclimatization. The experimental protocols were approved by the Ethical Committee of the Medical University in Lublin (license numbers 64/2007 and 69/2009). All procedures were in strict compliance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs
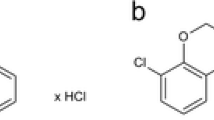
Sildenafil citrate (kindly provided by Polpharma S.A., Starogard Gdański, Poland), scopolamine methyl bromide (Sigma, Steinheim, Germany) and amitriptyline hydrochloride (kindly provided by ICN Polfa Rzeszów, Rzeszów, Poland) were dissolved in saline and administered 30 min before the respective test. Desipramine (Petylyl, AWD.pharma GmbH & Co. KG, Dresden, Germany) was suspended in a 0.5% aqueous solution of methyl cellulose (Sigma, Steinheim, Germany) and administered 60 min prior to the tests. All solutions and suspensions were prepared freshly and administered intraperitoneally (i.p.) in a volume of 0.1 ml per 10 g body weight. Control animals received an i.p. injection of a respective vehicle. The doses and pretreatment schedules were selected based on those reported in the literature and previous experiments in our laboratory (Karolewicz and Paul 2001; Kurt et al. 2004; Ushijima et al. 2005; Nieoczym et al. 2010a, b).
Forced swim test
The test was conducted according to the method described by Porsolt et al. (1977). Mice were placed individually into glass cylinders (height 25 cm, diameter 10 cm). The cylinders contained 10 cm of water maintained at temperature 23–25°C. Animals were allowed to swim for 6 min. The total duration of immobility was recorded during the last 4 min of the test. The duration of immobility was defined as the time when the mouse remained floating passively, made no attempts to escape and showed only slow movements to keep its head above the water.
Locomotor activity
The actimeter consists of a cylinder (30 cm diameter, 12 cm high, MultiServ, Lublin, Poland) equipped with two perpendicular infrared light beams located 1.5 cm above the floor. Mice were i.p. pretreated with respective drugs or drug combinations and after a given time period they were placed in the actimeter, and locomotor activity (number of interruptions of light beams) was recorded for a period of 10 min after placing the mouse into the actimeter.
Amitriptyline determination
Serum and brain concentrations of amitriptyline were determined by high performance liquid chromatography (HPLC) method. After drug pretreatment, mice were sacrificed by decapitation. The trunk blood was collected into polyethylene tubes. Serum was isolated 1h after blood coagulation by centrifugation at 5,000×g for 10 min at 4°C and frozen at −30°C. The brains were rapidly removed after decapitation, washed in saline and frozen on dry ice.
200 μl of serum was mixed with 10 μl of imipramine solution in methanol as an internal standard. The samples were alkalized with 200 μl of 2M sodium hydroxide and extracted with 3 ml of ethyl acetate:hexane (30:70, v/v). Likewise, 1 ml of brain homogenate was mixed with 20 μl of imipramine solution, alkalized with 500 μl of 2 M sodium hydroxide and extracted with 5 ml of dichloromethane:hexane:isoamyl alcohol (40:60:1, v/v/v). All samples were then shaken for 20 min (IKA VXR Vibrax, Germany). After centrifugation (1,500×g for 15 min), the organic layer was transferred to a new tube, and evaporated to dryness at 37°C under a stream of nitrogen. The residue was dissolved in 100 μl of methanol and 50 μl of this solution was injected into the HPLC system.
The HPLC system (Thermo Separation Products, San Jose, CA, USA) consisted of a P100 isocratic pump, a Rheodyne 7125 injector (Rheodyne, Cotati, CA, USA) with a 50 μl sample loop, a UV100 Variable-wavelength UV/VIS detector, operating at 214 nm and a SP4400 (Thermo Separation Products, San Jose, CA, USA) integrator. All analysis were performed at ambient temperature (21°C) on a 250 × 4.6 mm LiChrospher®100 RP-18 column (Merck, Darmstadt, Germany) with 5 μm particles, protected with a guard column (4 x 4 mm) with the same packing material. The mobile phase consisted of acetonitrile:50 mM potassium dihydrogen phosphate pH 3.5 (40:60, v/v) and was pumped at a flow rate of 1 ml/min.
The calibration curves were linear in the tested concentration ranges. The assay was reproducible with low intra- and inter-day variation (coefficient of variation less than 10%). Serum and brain concentrations of amitriptyline were expressed in ng/ml and ng/g of fresh tissue, respectively.
Statistics
All results are presented as mean ± standard error of the mean (SEM). Data obtained in behavioral tests were evaluated using one-way analysis of variance (one-way ANOVA) followed by the Tukey’s post hoc test for multiple comparisons. Serum and brain concentrations of amitriptyline were compared by unpaired Student’s t test. Statistical significance was noted when p values were equal to or less than 0.05.
Results
Forced swim test
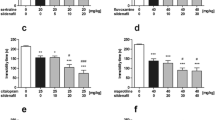
The first series of experiments was performed to evaluate the effect of sildenafil at doses of 1.25–20 mg/kg on immobility time in the forced swim test in mice. As shown in Fig. 1, sildenafil at any doses tested did not alter the total immobility duration as compared to the saline-treated group [ANOVA: F(5, 55) = 0.321, p = 0.898].
The effect of scopolamine (0.5 mg/kg) alone and in combination with sildenafil on immobility time in the forced swim test in mice is shown in Fig. 2 [ANOVA: F(4, 45) = 7.205, p < 0.001]. Scopolamine, as well as a combination of scopolamine with sildenafil at a dose of 1.25 mg/kg, did not alter the total immobility duration as compared to the control group (p > 0.05). However, joint administration of scopolamine with sildenafil at a dose of 2.5 mg/kg significantly reduced immobility time as compared to control group (p < 0.05) but not scopolamine-treated group (p < 0.05). Co-administration of scopolamine with sildenafil at the highest dose (5 mg/kg) caused further decrease in the total immobility duration (p < 0.001 vs. saline-treated group). Additionally, the Tukey's post hoc test revealed that the observed difference was statistically significant as compared to scopolamine-treated group (p < 0.01).
Effect of scopolamine administered alone and in combination with sildenafil on immobility time in the forced swim test in mice. Both drugs were administered i.p. 30 min before the test. Control animals received saline. Each experimental group consisted of 9–11 animals. Data are presented as mean + SEM. *p < 0.05, ***p < 0.001 as compared to saline-treated group; ## p < 0.01 as compared to scopolamine-treated group (one-way ANOVA followed by the Tukey’s post hoc test)
The influence of sildenafil on the antidepressant activity of amitriptyline (5 mg/kg) in the forced swim test in mice is shown in Fig. 3 [ANOVA: F(4, 49) = 82.355, p < 0.001]. Sildenafil at the lowest dose tested, i.e., 1.25 mg/kg, significantly enhanced the antidepressant activity of amitriptyline (p < 0.05). Higher doses of sildenafil (2.5 and 5 mg/kg) caused further increase in the anti-immobility action of amitriptyline (p < 0.001).
Effect of amitriptyline administered alone and in combination with sildenafil on immobility time in the forced swim test in mice. Both drugs were administered i.p. 30 min before the test. Control animals received saline. Each experimental group consisted of 9–12 animals. Data are presented as mean + SEM. ***p < 0.001 as compared to saline-treated group; # p < 0.05, ### p < 0.001 as compared to amitriptyline-treated group (one-way ANOVA followed by the Tukey’s post hoc test)
Figure 4 depicts the effect of joint administration of desipramine (20 mg/kg) and sildenafil on the immobility duration in mice [ANOVA: F(4, 69) = 9.095, p < 0.001]. Desipramine administered alone significantly reduced immobility time as compared to control group while sildenafil at any dose tested (5, 10 and 20 mg/kg) neither enhanced nor diminished the antidepressant activity of desipramine (p > 0.05 as compared to the desipramine-treated group).
Effect of desipramine administered alone and in combination with sildenafil on immobility time in the forced swim test in mice. Desipramine and sildenafil were administered i.p. 60 and 30 min before the test, respectively. Control animals received saline. Each experimental group consisted of 14–15 animals. Data are presented as mean + SEM. **p < 0.01, ***p < 0.001 as compared to control group (one-way ANOVA followed by the Tukey’s post hoc test)
Locomotor activity
The data obtained in spontaneous locomotor activity test are presented in Table 1. No significant alterations in locomotor activity with either drug or their combinations were demonstrated.
Pharmacokinetic studies
The effect of combined administration of amitriptyline and sildenafil on serum and brain amitriptyline concentrations in mice is shown in Table 2. Co-administration of sildenafil at a dose of 5 mg/kg with amitriptyline at a dose of 5 mg/kg did not change amitriptyline concentrations in serum and brain (t test: p = 0.472 and p = 0.978, respectively).
Discussion
Although sildenafil displays psychotropic action both in humans and animals (Milman and Arnold 2002; Kurt et al. 2004; Hotchkiss et al. 2005), its effect on the pathophysiology of depression remains unclear. There is no direct evidence that sildenafil may worsen or improve symptoms of depression (Tignol et al. 2004). It has been reported that sildenafil does not produce any effect on the behavioral response in the forced swim test in rodents when given alone (Almeida et al. 2006; Dhir and Kulkarni 2007a, b; Brink et al. 2008; Savegnago et al. 2008; Brocardo et al. 2008). However, it reversed the anti-immobility action of such substances like adenosine (Kaster et al. 2005b), memantine (Almeida et al. 2006), lithium (Ghasemi et al. 2008), dizocilpine (Dhir and Kulkarni 2008), venlafaxine (Dhir and Kulkarni 2007a), bupropion (Dhir and Kulkarni 2007b), escitalopram (Zomkowski et al. 2010), tramadol (Jesse et al. 2008), berberine chloride (Kulkarni and Dhir 2008), diphenyl diselenide (Savegnago et al. 2008), folic acid (Brocardo et al. 2008) and potassium channel inhibitors (Kaster et al. 2005a). In our study, sildenafil did not affect the immobility time which is in line with the above-mentioned reports. Interestingly, it exerted anti-immobility action after central muscarinic receptor blockade with scopolamine. Scopolamine administered alone did not affect animal behavior. Our results are consistent with findings of Brink et al. (2008). In their study, sildenafil given alone did not produce any effect on immobility time in the forced swim test in rats nor did it reverse the anti-immobility action of fluoxetine. Nonetheless, sildenafil administered chronically exerted the antidepressant effect after blocking muscarinic receptors with atropine. Moreover, in combination with atropine, sildenafil as well as tadalafil, a potent and more selective than sildenafil PDE5 inhibitor (Rosen and McKenna 2002), exerted antidepressant action in the forced swim test in FSL rats, which represent a genetic animal model of depression (Liebenberg et al. 2010a). Brink et al. (2008) also showed that sildenafil potentiates muscarinic acetylcholine receptor signaling capacity in cultured human neuroblastoma cells and they proposed that sildenafil possesses antidepressant properties which are attenuated or even lost because of its simultaneous ability to enhance cholinergic transmission. Cholinomimetic effects of sildenafil were also demonstrated by Patil et al. (2004). Herein we report that anti-immobility activity of sildenafil in mice can be revealed even after its single administration with the co-administration of scopolamine.
Sildenafil, which acts as a selective PDE5 inhibitor, elevates intracellular cGMP level (Rosen and McKenna 2002). Phosphodiesterases, cyclic nucleotide-gated channels and cGMP-dependent protein kinase (protein kinase-G) are the main downstream effectors of cGMP (Bryan et al. 2009). On the ground of the observation that inhibition of protein kinase-G abolished the anti-immobility effect of sildenafil, Liebenberg et al. (2010b) concluded that the cholinergic-cGMP-protein kinase-G interactions are implicated in the antidepressant action of sildenafil. It is worth mentioning that in the absence of atropine sildenafil at a lower dose of 3 mg/kg, but not at higher doses of 10 and 20 mg/kg, reduced immobility time and increased climbing behavior in FSL rats. This observation suggests the involvement of monoaminergic neurotransmission in the animal response to sildenafil treatment (Liebenberg et al. 2010a). PDE5 is widely distributed throughout the brain with varying regional expression. The highest PDE5 RNA level was found in cerebellum, medulla oblongata, substantia nigra and subthalamic nucleus (Loughney et al. 1998; van Staveren et al. 2004). For this reason, inhibition of PDE by sildenafil may bring about various effects including modulation of serotonergic, noradrenergic, glutamatergic or cholinergic neurotransmitter systems. Supposing that sildenafil enhanced the cholinergic neurotransmission in these brain areas which are involved in pathophysiology of depression (e.g., hippocampus), it may induce depressogenic effects (Liebenberg et al. 2010b). Conversely, in the presence of an antimuscarinic agent, antidepressant properties of sildenafil may be revealed.
In view of the above-mentioned reports, we assumed that sildenafil may augment the activity of these antidepressant drugs which exhibits cholinolytic action. The marked anticholinergic activity is a feature of many of the tricyclic antidepressants and muscarinic receptors blockade results in unwanted side effects including xerostomia, constipation, tachycardia and blurred vision. There is no clear-cut evidence that antimuscarinic effects of antidepressant contribute to their clinical efficacy. Thus, drugs devoid of the effects in question were highly desirable (Szabadi et al. 1980; Richelson 2001; Nelson 2009). Amitriptyline is one of the most widely used tricyclic antidepressants, which shows properties of a non-selective muscarinic receptors antagonist (Snyder and Yamamura 1977; McKinney et al. 1988). Its affinity for these receptors is approximately one-tenth of the affinity of atropine (Brunton et al. 2008). The results obtained in the forced swim test confirmed our assumption. Sildenafil at relatively low doses caused a potent increase in the antidepressant activity of amitriptyline. Interestingly, the highest dose of sildenafil (5 mg/kg) caused over 90% increase of the anti-immobility action of amitriptyline. The observed changes in immobility time were not due to the changes in spontaneous locomotor activity as combined administration of amitriptyline and sildenafil did not affect motor performance in mice. The enhancement of antidepressant activity of amitriptyline caused by concomitant treatment with sildenafil may have either a pharmacodynamic or pharmacokinetic basis. To evaluate the potential pharmacokinetic interaction between amitriptyline and sildenafil, brain and serum amitriptyline concentrations were analyzed. The results indicate that the effect observed in the forced swim test was related to pharmacodynamic rather than pharmacokinetic interaction because sildenafil did not modify amitriptyline concentrations in both examined tissues.
To establish whether the behavioral interactions between sildenafil and amitriptyline were specifically related to cholinolytic properties of amitriptyline, we investigated the effect of sildenafil on the antidepressant activity of desipramine. Among all tricyclics, desipramine is the least potent anticholinergic agent (Nelson 2009). As it might have been expected, sildenafil did not affect the anti-immobility action of desipramine in the forced swim test in mice, even though it was administered at higher doses than in combination with amitriptyline. Because no alteration in the antidepressant activity of desipramine after joint administration with sildenafil was observed, there were no rationale for assessing desipramine concentrations in mice serum and brain tissue.
In summary, the present study demonstrates for the first time that sildenafil enhanced the antidepressant activity of amitriptyline in the forced swim test in mice, and this effect was not due to a pharmacokinetic interaction. In addition, our results constitute further support for the above-mentioned thesis concerning cholinomimetic action of sildenafil and suggest that sildenafil may augment the activity of antidepressant drugs which exhibit antimuscarinic properties. Further studies, with the use of other animal models, are recommended to better understanding of the observed phenomenon.
References
Almeida RC, Felisbino CS, Lopez MG, Rodrigues AL, Gabilan NH (2006) Evidence for the involvement of l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of memantine in mice. Behav Brain Res 168:318–322
Ansorge MS, Hen R, Gingrich JA (2007) Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol 7:8–17
Brink CB, Clapton JD, Eagar BE, Harvey BH (2008) Appearance of antidepressant-like effect by sildenafil in rats after central muscarinic receptor blockade: evidence from behavioural and neuro-receptor studies. J Neural Transm 115:117–125
Brocardo PS, Budni J, Lobato KR, Kaster MP, Rodrigues AL (2008) Antidepressant-like effect of folic acid: Involvement of NMDA receptors and l-arginine-nitric oxide-cyclic guanosine monophosphate pathway. Eur J Pharmacol 598:37–42
Brunton L, Parker K, Blumenthal D, Buxton I (2008) Muscarinic receptor agonists and antagonists. In: Brunton L, Parker K, Blumenthal D, Buxton I (eds) Goodman and Gilman’s manual of pharmacology and therapeutics. The McGrow-Hill Companies, New York, pp 114–125
Bryan NS, Bian K, Murad F (2009) Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci 14:1–18
Chau D, Rada PV, Kosloff RA, Hoebel BG (1999) Cholinergic, M1 receptors in the nucleus accumbens mediate behavioral depression. A possible downstream target for fluoxetine. Ann N Y Acad Sci 877:769–774
Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG (2001) Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience 104:791–798
Dhir A, Kulkarni SK (2007a) Involvement of l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of venlafaxine in mice. Prog Neuropsychopharmacol Biol Psychiatry 31:921–925
Dhir A, Kulkarni SK (2007b) Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol 568:177–185
Dhir A, Kulkarni SK (2008) Possible involvement of nitric oxide (NO) signaling pathway in the antidepressant-like effect of MK-801 (dizocilpine), a NMDA receptor antagonist in mouse forced swim test. Indian J Exp Biol 46:164–170
Dilsaver SC (1986) Cholinergic mechanisms in depression. Brain Res 396:285–316
Dilsaver SC, Snider RM, Alessi NE (1986) Stress induces supersensitivity of a cholinergic system in rats. Biol Psychiatry 21:1093–1096
Drevets WC, Furey ML (2010) Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry 67:432–438
Furey ML, Drevets WC (2006) Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63:1121–1129
Ghasemi M, Sadeghipour H, Mosleh A, Sadeghipour HR, Mani AR, Dehpour AR (2008) Nitric oxide involvement in the antidepressant-like effects of acute lithium administration in the mouse forced swimming test. Eur Neuropsychopharmacol 18:323–332
Ghofrani HA, Osterloh IH, Grimminger F (2006) Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov 5:689–702
Hotchkiss AK, Pyter LM, Gatien ML, Wen JC, Milman HA, Nelson RJ (2005) Aggressive behavior increases after termination of chronic sildenafil treatment in mice. Physiol Behav 83:683–688
Jabbi M, Korf J, Ormel J, Kema IP, den Boer JA (2008) Investigating the molecular basis of major depressive disorder etiology: a functional convergent genetic approach. Ann N Y Acad Sci 1148:42–56
Janowsky DS, el Yousef MK, Davis JM, Sekerke HJ (1972) A cholinergic–adrenergic hypothesis of mania and depression. Lancet 2:632–635
Janowsky DS, el Yousef MK, Davis JM (1974) Acetylcholine and depression. Psychosom Med 36:248–257
Jesse CR, Bortolatto CF, Savegnago L, Rocha JB, Nogueira CW (2008) Involvement of l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of tramadol in the rat forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry 32:1838–1843
Karolewicz B, Paul IA (2001) Group housing of mice increases immobility and antidepressant sensitivity in the forced swim and tail suspension tests. Eur J Pharmacol 415:197–201
Kaster MP, Ferreira PK, Santos AR, Rodrigues AL (2005a) Effects of potassium channel inhibitors in the forced swimming test: possible involvement of l-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav Brain Res 165:204–209
Kaster MP, Rosa AO, Santos AR, Rodrigues AL (2005b) Involvement of nitric oxide-cGMP pathway in the antidepressant-like effects of adenosine in the forced swimming test. Int J Neuropsychopharmacol 8:601–606
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Kulkarni SK, Dhir A (2008) On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol 589:163–172
Kurt M, Bilge SS, Aksoz E, Kukula O, Celik S, Kesim Y (2004) Effect of sildenafil on anxiety in the plus-maze test in mice. Pol J Pharmacol 56:353–357
Liebenberg N, Harvey BH, Brand L, Brink CB (2010a) Antidepressant-like properties of phosphodiesterase type 5 inhibitors and cholinergic dependency in a genetic rat model of depression. Behav Pharmacol 21:540–547
Liebenberg N, Wegener G, Harvey BH, Brink CB (2010b) Investigating the role of protein kinase-G in the antidepressant-like response of sildenafil in combination with muscarinic acetylcholine receptor antagonism. Behav Brain Res 209:137–141
Loughney K, Hill TR, Florio VA, Uher L, Rosman GJ, Wolda SL, Jones BA, Howard ML, McAllister-Lucas LM, Sonnenburg WK, Francis SH, Corbin JD, Beavo JA, Ferguson K (1998) Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3′, 5′-cyclic nucleotide phosphodiesterase. Gene 216:139–147
Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J (2007) Neurobiology of depression: an integrated view of key findings. Int J Clin Pract 61:2030–2040
Manji HK, Drevets WC, Charney DS (2001) The cellular neurobiology of depression. Nat Med 7:541–547
McKinney M, Lee NH, Anderson DJ, Vella-Rountree L, el Fakahany EE (1988) Non-selectivity of amitriptyline for subtypes of brain muscarinic receptors demonstrated in binding and functional assays. Eur J Pharmacol 157:51–60
Mearns J, Dunn J, Lees-Haley PR (1994) Psychological effects of organophosphate pesticides: a review and call for research by psychologists. J Clin Psychol 50:286–294
Meyerson LR, Wennogle LP, Abel MS, Coupet J, Lippa AS, Rauh CE, Beer B (1982) Human brain receptor alterations in suicide victims. Pharmacol Biochem Behav 17:159–163
Milman HA, Arnold SB (2002) Neurologic, psychological, and aggressive disturbances with sildenafil. Ann Pharmacother 36:1129–1134
Mineur YS, Picciotto MR (2010) Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31:580–586
Murray CJ, Lopez AD (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349:1498–1504
Nelson CJ (2009) Tricyclic and tetracyclic drugs. In: Schatzberg AF, Nemeroff CB (eds) The American psychiatric publishing textbook of psychopharmacology. American Psychiatric Publishing, Arlington, pp 263–288
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Nieoczym D, Łuszczki JJ, Czuczwar SJ, Wlaź P (2010a) Effect of sildenafil on the anticonvulsant action of classical and second-generation antiepileptic drugs in maximal electroshock-induced seizures in mice. Epilepsia 51:1552–1559
Nieoczym D, Socała K, Rundfeldt C, Wlaź P (2010b) Effects of sildenafil on pentylenetetrazol-induced convulsions in mice and amygdala-kindled seizures in rats. Pharmacol Rep 62:383–391
Nurnberg HG, Hensley PL (2003) Sildenafil citrate for the management of antidepressant-associated erectile dysfunction. J Clin Psychiatry 64(Suppl 10):20–25
Overstreet DH, Russell RW (1982) Selective breeding for diisopropyl fluorophosphate-sensitivity: behavioural effects of cholinergic agonists and antagonists. Psychopharmacology (Berl) 78:150–155
Overstreet DH, Friedman E, Mathe AA, Yadid G (2005) The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev 29:739–759
Pacher P, Kecskemeti V (2004) Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Curr Med Chem 11:925–943
Patil CS, Jain NK, Singh VP, Kulkarni SK (2004) Cholinergic-NO-cGMP mediation of sildenafil-induced antinociception. Indian J Exp Biol 42:361–367
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Richelson E (2001) Pharmacology of antidepressants. Mayo Clin Proc 76:511–527
Rosen RC, McKenna KE (2002) PDE-5 inhibition and sexual response: pharmacological mechanisms and clinical outcomes. Annu Rev Sex Res 13:36–88
Savegnago L, Jesse CR, Pinto LG, Rocha JB, Barancelli DA, Nogueira CW, Zeni G (2008) Diphenyl diselenide exerts antidepressant-like and anxiolytic-like effects in mice: involvement of l-arginine-nitric oxide-soluble guanylate cyclase pathway in its antidepressant-like action. Pharmacol Biochem Behav 88:418–426
Snyder SH, Yamamura HI (1977) Antidepressants and the muscarinic acetylcholine receptor. Arch Gen Psychiatry 34:236–239
Szabadi E, Gaszner P, Bradshaw CM (1980) The peripheral anticholinergic activity of tricyclic antidepressants: comparison of amitriptyline and desipramine in human volunteers. Br J Psychiatry 137:433–439
Tignol J, Furlan PM, Gomez-Beneyto M, Opsomer R, Schreiber W, Sweeney M, Wohlhuter C (2004) Efficacy of sildenafil citrate (Viagra) for the treatment of erectile dysfunction in men in remission from depression. Int Clin Psychopharmacol 19:191–199
Ushijima K, Sakaguchi H, Sato Y, To H, Koyanagi S, Higuchi S, Ohdo S (2005) Chronopharmacological study of antidepressants in forced swimming test of mice. J Pharmacol Exp Ther 315:764–770
Uthayathas S, Karuppagounder SS, Thrash BM, Parameshwaran K, Suppiramaniam V, Dhanasekaran M (2007) Versatile effects of sildenafil: recent pharmacological applications. Pharmacol Rep 59:150–163
van Staveren WC, Steinbusch HW, Markerink-van Ittersum M, Behrends S, de Vente J (2004) Species differences in the localization of cGMP-producing and NO-responsive elements in the mouse and rat hippocampus using cGMP immunocytochemistry. Eur J Neurosci 19:2155–2168
Zomkowski AD, Engel D, Gabilan NH, Rodrigues AL (2010) Involvement of NMDA receptors and l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effects of escitalopram in the forced swimming test. Eur Neuropsychopharmacol 20:793–801
Acknowledgments
This study was supported by Funds for Statutory Activity of Maria Curie-Skłodowska University, Lublin, Poland. The authors wish to thank Polpharma S.A. (Starogard Gdański, Poland) and ICN Polfa Rzeszów (Rzeszów, Poland) for generous gifts of sildenafil and amitriptyline, respectively.
Conflict of interest
The authors declare they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Socała, K., Nieoczym, D., Wyska, E. et al. Sildenafil, a phosphodiesterase type 5 inhibitor, enhances the antidepressant activity of amitriptyline but not desipramine, in the forced swim test in mice. J Neural Transm 119, 645–652 (2012). https://doi.org/10.1007/s00702-011-0756-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-011-0756-9