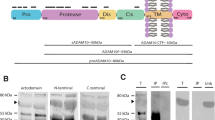
Abstract
Decreased levels of β-amyloid (Aβ) 1-42 in cerebrospinal fluid (CSF) are characteristic for Alzheimer’s disease (AD) and are also evident in Creutzfeldt–Jakob disease (CJD). Aβ plaques are thought to be responsible for this decrease in AD patients, whereas such Aβ plaques are rarely seen in CJD. To investigate the Aβ pattern in brain and CSF of neuropathologically confirmed CJD and AD patients we used an electrophoretic method to investigate Aβ peptide fractions which are not accessible to ELISA and immunohistochemistry. We analyzed Aβ peptides in the CSF of autopsy-confirmed CJD and AD patients and the corresponding brain homogenates using a quantitative urea-based Aβ electrophoresis immunoblot (Aβ-SDS-PAGE/immunoblot).The CSF Aβ1-42 decrease correlated with the brain Aβ load in AD, but not in CJD. There was no difference in the soluble fractions of brain homogenate in AD and CJD. We therefore conclude that different mechanisms in AD and CJD are responsible for the Aβ1-42 decrease in the CSF.

Similar content being viewed by others
References
Bibl M, Esselmann H, Otto M, Lewczuk P, Cepek L, Ruther E, Kornhuber J, Wiltfang J (2004) Cerebrospinal fluid amyloid beta peptide patterns in Alzheimer’s disease patients and nondemented controls depend on sample pretreatment: indication of carrier-mediated epitope masking of amyloid beta peptides. Electrophoresis 25:2912–2918
Bibl M, Mollenhauer B, Esselmann H, Lewczuk P, Klafki HW, Sparbier K, Smirnov A, Cepek L, Trenkwalder C, Ruther E, Kornhuber J, Otto M, Wiltfang J (2006) CSF amyloid-beta-peptides in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease dementia. Brain 129:1177–1187
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Citron M, Diehl TS, Gordon G, Biere AL, Seubert P, Selkoe DJ (1996) Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities. Proc Natl Acad Sci USA 93:13170–13175
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 59:512–519
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Glenner GG, Wong CW, Quaranta V, Eanes ED (1984) The amyloid deposits in Alzheimer’s disease: their nature and pathogenesis. Appl Pathol 2:357–369
Haass C, Hung AY, Schlossmacher MG, Oltersdorf T, Teplow DB, Selkoe DJ (1993) Normal cellular processing of the beta-amyloid precursor protein results in the secretion of the amyloid beta peptide and related molecules. Ann N Y Acad Sci 695:109–116
Hainfellner JA, Wanschitz J, Jellinger K, Liberski PP, Gullotta F, Budka H (1998) Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 96:116–122
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Jarrett JT, Berger EP, Lansbury PT Jr (1993) The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32:4693–4697
Krasemann S, Groschup MH, Harmeyer S, Hunsmann G, Bodemer W (1996) Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol Med 2:725–734
Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J (1996) Diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Arch Neurol 53:913–920
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R et al (1995) Reduction of beta-amyloid peptide 42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol 38:643–648
Otto M, Esselmann H, Schulz-Shaeffer W, Neumann M, Schroter A, Ratzka P, Cepek L, Zerr I, Steinacker P, Windl O, Kornhuber J, Kretzschmar HA, Poser S, Wiltfang J (2000) Decreased beta-amyloid1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology 54:1099–1102
Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, Baybutt HN, Turner AJ, Hooper NM (2007) Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc Natl Acad Sci USA 104:11062–11067
Pitschke M, Prior R, Haupt M, Riesner D (1998) Detection of single amyloid beta-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat Med 4:832–834
Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, Giese A, Groschup MH, Kretzschmar HA (2000) The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am J Pathol 156:51–56
Schwarze-Eicker K, Keyvani K, Gortz N, Westaway D, Sachser N, Paulus W (2005) Prion protein (PrPc) promotes beta-amyloid plaque formation. Neurobiol Aging 26:1177–1182
Tapp PD, Siwak CT, Gao FQ, Chiou JY, Black SE, Head E, Muggenburg BA, Cotman CW, Milgram NW, Su MY (2004) Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J Neurosci 24:8205–8213
WHO (1998) Report of a WHO consultation on global surveillance, diagnosis and therapy of human transmissible spongiform encephalopathies. Geneva: WHO; 9–11 February. WHO/EMC/ZDI/98.9
Wiltfang J, Esselmann H, Cupers P, Neumann M, Kretzschmar H, Beyermann M, Schleuder D, Jahn H, Ruther E, Kornhuber J, Annaert W, De Strooper B, Saftig P (2001) Elevation of beta-amyloid peptide 2-42 in sporadic and familial Alzheimer’s disease and its generation in PS1 knockout cells. J Biol Chem 276:42645–42657
Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, Paul S, Schmidt B, Klafki HW, Maler M, Dyrks T, Bienert M, Beyermann M, Ruther E, Kornhuber J (2002) Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem 81:481–496
Wiltfang J, Esselmann H, Smirnov A, Bibl M, Cepek L, Steinacker P, Mollenhauer B, Buerger K, Hampel H, Paul S, Neumann M, Maler M, Zerr I, Kornhuber J, Kretzschmar HA, Poser S, Otto M (2003) Beta-amyloid peptides in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Ann Neurol 54:263–267
Acknowledgments
This study was supported in part by a grant from the Bundesministerium fuer Gesundheit und soziale Sicherung and by the Bundesministerium fuer Forschung und Technik (MO,HAK, SR). BM is supported by the Stifterverband der Deutschen Wissenschaft (S134-10.008) and the Michael J. Fox Foundation for Parkinson’s Research. MB, HE, MO and JW are supported by grants from the German Federal Ministry of Education and Research (Competence Net Dementia, grant O1 GI 0420), MB was supported by the Research program, Faculty of Medicine, Georg-August-Universität Göttingen; JW is supported by grants from the German Federal Ministry of Education and Research CJK (01 GI 0301) and HBPP-NGFN2 (01 GR 0447). The authors would like to thank Birgit Otte and Heike Zech for excellent technical assistance. Brain samples from subjects were recruited by the ‘Brain-Net’ (supported by the German Federal Ministry of Education and Research BMBF).
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Mollenhauer and H. Esselmann contributed equally to this work.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00702-011-0669-7
Rights and permissions
About this article
Cite this article
Mollenhauer, B., Esselmann, H., Roeber, S. et al. Different CSF β-amyloid processing in Alzheimer’s and Creutzfeldt–Jakob disease. J Neural Transm 118, 691–697 (2011). https://doi.org/10.1007/s00702-010-0543-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0543-z