Abstract
Tofisopam is a member of the 2,3-benzodiazepine compound family which is marketed for the treatment of anxiety in some European countries. In contrast to classical 1,4-benzodiazepines, the compound does not bind to the benzodiazepine binding site of the γ-aminobutyric acid receptor and its psychopharmacological profile differs from such compounds. In addition to anxiolytic properties, antipsychotic effects are reported. We now show that tofisopam, 50 mg/kg intraperitoneally (i.p.), administered in parallel to repeated doses of dizocilpine 0.2 mg/kg i.p. can ameliorate dizocilpine-induced prolongation of immobility, which is considered to be a model of negative symptoms of psychosis. We further show that tofisopam acts as an isoenzyme-selective inhibitor of phosphodiesterases (PDEs) with highest affinity to PDE-4A1 (0.42 μM) followed by PDE-10A1 (0.92 μM), PDE-3 (1.98 μM) and PDE-2A3 (2.11 μM). The data indicate that tofisopam is an interesting candidate for the adjuvant treatment of psychosis with focus on negative symptoms. Combined partial inhibition of PDE-4 and PDE-10 as well as PDE-2 may be the underlying mechanism to this activity. Due to the good safety profile of tofisopam as evident from long-term use of this agent in patients, it may be concluded that dual or triple inhibition of PDE isoenzymes with additive or synergistic effects may be an interesting approach to pharmacological activity, resulting in active compounds with beneficial safety profile. Dose-limiting side effects such as emesis induced by selective inhibition of PDE-4 may be prevented by such strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tofisopam, a 2,3-benzodiazepine (2,3-BDZs), represent a unique drug among CNS-active compounds. Though sharing a common chemical backbone to ‘classical’ 1,4-benzodiazepines and though sharing the anxiolytic activity, the compound does not interact with the benzodiazepine binding site of the γ-aminobutyric acid (GABA) receptor (Petocz 1993). It is an anxiolytic without sedative–hypnotic or muscle relaxant effects. Tofisopam does not impair psychomotor and intellectual performance, like other benzodiazepines do. In contrast, it has a mild cognitive stimulatory activity. It is also potent in alleviating vegetative symptoms accompanying anxiety disorders (Szegő et al. 1993). Tofisopam does not possess anticonvulsive properties and does not induce sleep even in subtoxic doses, and only such subtoxic doses enhance the effect of barbiturates and ethanol. Applying doses above 200 mg/kg to experimental animals, tofisopam exhibits effects similar to that of neuroleptics (e.g., catalepsy, ptosis, decrease of pentylenetetrazole threshold, potentiation of amphetamine- and apomorphine-induced stereotypy) (Petocz 1993). This unique pharmacological profile lead to the conclusion that tofisopam may be of interest for treatment of psychosis. Current antipsychotics are well capable of ameliorating the positive symptoms of a psychotic episode. If administered chronically, the re-occurrence of such an episode can be retarded or even prevented. However, the treatment with classical antipsychotic is hampered with side effects and patient compliance for long-term treatment is often low, potentially due to these side effects. Some of the side effects of such classical antipsychotics overlap with the negative symptoms of psychosis, i.e., the compounds exert negative effects on attention, concentration, cognition and memory (Arnt and Skarsfeldt 1998).
While some 2,3-BDZs and in part also tofisopam have been shown to induce antipsychotic-like effects in classical animal models of psychosis linked to the positive symptoms of the disease, i.e., in the apomorphine climbing test, the conditioned avoidance reaction and—to a lesser extent—in the catalepsy test, the pharmacological profile and the side effect profile of tofisopam in man differs from classical antipsychotics (Horváth et al. 2000). Due to the reported mild stimulatory activity (Szegő et al. 1993) which is in contrast to the activity of classical antipsychotics we were interested to see whether tofisopam may be active in a model of negative symptoms of psychosis, which mimics the state of avolition. A respective model has been described by Noda et al. (1995).
Due to the interesting pharmacological profile of tofisopam we were also interested to learn more about the mechanism of action. Early binding studies identified specific and exclusive binding of 2,3-BDZs within the striatum and associated structures (Horváth et al. 2000). The binding site was named girisopam binding site, since this 2,3-BDZ derivative was found to have the highest affinity. The distribution of this proposed binding site overlaps largely with the distribution of the phosphodiesterase 10 isoenzyme (PDE-10), which is highly and selectively expressed in striatum (Seeger et al. 2003). Furthermore, PDE-10 inhibitors are discussed to be interesting targets for antipsychotic agents (Siuciak et al. 2006). Horváth et al. (2000) proposed that the mechanism of action of tofisopam may be related to alteration in the phosphorylation of protein(s). While inhibition of phosphodiesterase does not directly result in protein phosphorylation, the second messengers—cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP)—which are cleaved by phosphodiesterases, can activate protein kinases resulting in increased protein phosphorylation. For these reasons we screened different PDE isoenzymes including PDE-10 for specific interaction with tofisopam.
Materials and methods
Phosphodiesterase screen
To screen for interaction with different PDE isoenzymes, tofisopam was analyzed using the IMAP Technology (Molecular Devices Inc., Sunnyvale, CA, USA), as established at Scottish Biomedical, Glasgow, UK. In brief, the assay is based on the high-affinity binding of phosphate by immobilized metal coordination complexes on nanoparticles. The binding reagent complexes with phosphate groups on nucleotide monophosphate generated from cyclic nucleotides (cAMP/cGMP) through phosphodiesterases. With fluorescence polarization detection, binding causes a change in the rate of the molecular motion of the phosphate-bearing molecule and results in an increase in the fluorescence polarization value observed for the fluorescent label attached to the substrate. Using human recombinant protein of PDE-1A3, PDE-2A3, PDE-3A, PDE-4A1, PDE-5 (catalytic domain only), PDE-6C, PDE-8A1, PDE-9A1, PDE-10A1 and PDE-11A1, selective inhibition of different isoenzymes was screened using a single-point assay at a concentration of 10,000 nM. The assays have been validated using reference PDE inhibitors (MacKenzie et al. 2010). Tofisopam was dissolved in DMSO and diluted in assay buffer, final DMSO concentration 0.1%. Reactions were carried out in duplicate. For isoenzymes where the single-point analysis resulted in an inhibition >50%, IC50 was determined using the same method but with an 8-point assay, using 3 parallel experiments for each isoenzyme. The IC50 determination was conducted for PDE-2A3, PDE-3A, PDE-4A1 and PDE-10A1.
Animals
The animal experiments were carried out on a total of 49 male Albino Swiss mice weighing 25–30 g purchased from the licensed breeder (Laboratory Animals Breeding, Górzkowska, Warsaw, Poland). They were used in this study after at least 1 week of acclimatization. The animals were housed in polycarbonate cages in groups of 6–10 under the strictly controlled laboratory conditions (ambient temperature 22–23°C, relative humidity about 45–55%, 12/12 light/dark cycle, light on at 6:00 h; chow pellets and tap water continuously available). The experimental protocol was approved by the Local Ethics Committee at the Medical University of Lublin (license no. 18/2007), and all the procedures were in strict compliance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs
Tofisopam [1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine, racemic mixture, kindly provided by EGIS Pharmaceuticals PLC (Budapest, Hungary)], was freshly suspended in a 0.5% aqueous solution of methyl cellulose (Sigma, St. Louis, MO, USA) and administered intraperitoneally (i.p.) at a volume of 5 ml/kg. Dizocilpine [(+)-5-methyl-10,11-dihydro-H-dibenzocyclohepten-5,10-imine hydrogen maleate, MK-801; purchased from Sigma] was dissolved extempore in physiological saline and administered i.p. at a volume of 5 ml/kg. Control animals received respective vehicles at appropriate volumes.
Forced swimming test in mice chronically treated with dizocilpine
Acute administration of the potent N-methyl-d-aspartate (NMDA) receptor antagonist phencyclidine can induce both, positive and negative symptoms of psychosis in man and exacerbates psychosis in schizophrenic patients (Abdel-Naby et al. 2001). In animals, phencyclidine can induce a wide range of abnormal behaviors which may be linked to psychotic behavior. Upon repeated administration of phencyclidine, a state of avolition can be induced in mice, which persists for several days after termination of treatment with phencyclidine. Avolition is one of the negative symptoms of schizophrenia; it is quantified in a forced swim test and animals exhibit a state of prolonged immobility as indicator of avolition (Abdel-Naby et al. 2001). This model was proposed to be an ideal model of negative symptoms of psychosis (Noda et al. 1995), it is however limited to the depressive component and may not be useful to model other negative symptoms such as learning and memory deficits or social retract.
Since phencyclidine is a controlled substance, we have re-established the model using the NMDA-receptor antagonist dizocilpine instead. The forced swim test and the drug treatment protocol were carried out on mice essentially as described by Noda et al. (1995). The dose of dizocilpine and the treatment schedule was selected based on preliminary experiments (data not presented). On the first day (Friday), each animal was placed individually into a glass cylinder (height 20 cm, diameter 15 cm) containing 11 cm of water maintained at 23°C, and was forced to swim for 3 min, and the immobility time was recorded (first measurement of immobility as baseline measurement). The mouse was judged to be immobile when it remained floating passively in the water. For induction of avolition, mice were chronically treated with 0.2 mg/kg dizocilpine i.p. daily excluding weekends starting on the fourth day (Monday) after the first swim test, until day 19 (Friday). After a 2-day drug-free period, on the 22nd day (Monday), each mouse was placed in water again for 3 min, and the immobility was recorded to test for prolongation of immobility (second measurement of immobility).
For the current experiment, a group of 49 mice was exposed to an initial swim test to determine the individual immobility time. The mice were thereafter randomized to three treatment groups of 16–17 mice. Three days after this baseline swim test, treatment was initiated. One group received the vehicle of dizocilpine and vehicle of tofisopam (vehicle control group); the second group received treatment with dizocilpine 0.2 mg/kg once daily plus tofisopam-vehicle twice daily on 5 days per week (psychosis control), and a third group dizocilpine 0.2 mg/kg once daily plus tofisopam 50 mg/kg twice daily on 5 days per week until experimental day 19. The treatment schedule consisted of two administrations of tofisopam at a dose of 50 mg/kg i.p. each, given 4 h apart. The first dose was given 10 min before the daily dizocilpine dose. The 4-h break between two tofisopam administrations was selected based on the rapid absorption and short half-life of tofisopam (Klebovich and Abermann 1993) to safely cover the duration of action of dizocilpine. After a washout of 2 days, a second quantification of immobility was conducted on day 22, about 72 h after the last dizocilpine dose and 68 h after the last tofisopam dose. This washout period was selected to ensure that animals are free of both, tofisopam and dizocilpine plasma levels. The half-life of dizocilpine in rodents is reported to be in the range of 2 h (Vezzani et al. 1989).
Statistical analysis
All data are presented as means ± standard error of the mean (SEM), where appropriate. Statistical analysis of the effect of tofisopam on the prolonged immobility after termination of treatment was performed by one-way analysis of variance (ANOVA) of the individual differences between the two measurements of immobility followed by Tukey’s post hoc test. The use of individual differences results in a baseline adjustment. A p value less than or equal to 0.05 was considered statistically significant.
To test for effects of repeated determination of immobility in the same group, individual groups were also compared using paired t test.
Results
Phosphodiesterase screen (Table 1)
Tofisopam was found to act as low-affinity blocker of a number of PDE isoenzymes. While no interaction was found with PDE-6, PDE-8, PDE-9, and PDE-11, weak inhibition was identified for PDE-1 and PDE-5. Due to an inhibition of less than 50% measured at 10,000 μM, no IC50 was determined for these isoenzymes. Inhibition in the high nanomolar to low micromolar range was found for PDE-2A3 (2.11 ± 1.8 μM), PDE-3A (1.98 ± 1.7 μM), PDE-4A1 (0.42 ± 0.8 μM), and PDE-10A1 (0.92 ± 1.2 μM).
Effects of tofisopam in a model of negative symptoms of psychosis (Fig. 1)
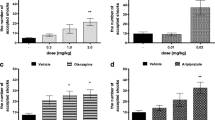
The animals of all three treatment groups had similar immobility times at the pre-test. In addition, the immobility time of vehicle-treated animals was compared between the pre-test and the second measurement on day 22 and the immobility time was similar for both assessments, with 95.2 s at the first measurement and 103.2 at the second measurement (not significant, paired t test), indicating that repeated measurement of immobility had no effect on immobility time. As expected, animals which had received repeated doses of dizocilpine showed a significant prolongation of the immobility time, with 92.0 s at the first test and 128.2 s at the second test. This difference was highly significant (p < 0.001, paired t test) and the data indicate that the model could be established successfully.
Immobility of mice in the forces swim test prior to repeated administration of dizocilpine (0.2 mg/kg i.p. once daily 5 days per week) or dizocilpine plus tofisopam, 50 mg/kg i.p. twice daily, 5 days per week (panel a) and on day 22, 3 days after cessation of drug treatment (panel b). Immobility on day 1 was similar for all three groups [ANOVA: F(2, 46) = 0.2696, not significant]. On day 21 (panel b), immobility of the dizocilpine-treated mice was significantly increased if compared to the control group; ANOVA on individual differences: F(2, 46) = 6.005, p = 0.0048. The immobility of the tofisopam-treated group was not significantly different from the vehicle group. **p < 0.004 versus respective control group (Tukey’s post hoc test)
To evaluate the effect of subchronic treatment of tofisopam on the avolition representing as prolonged immobility induced by repeated administration of dizocilpine, the individual differences of the immobility times determined during the first measurement on day 1 and second measurement on day 22 were calculated and compared using one-way analysis of variance (ANOVA). It became evident that treatment with tofisopam in parallel to treatment with dizocilpine ameliorated the prolonged immobility time. The immobility of this group amounted to 117 s and was not significantly different from the vehicle control group. In contrast, the immobility of the mice treated with dizocilpine was significantly prolonged [ANOVA on individual differences: F(2, 46) = 6.005, p = 0.0048; Tukey’s post hoc test: p < 0.004].
Discussion
Tofisopam is an atypical 2,3-benzodiazepine which does not bind to benzodiazepine receptors and which is devoid of CNS side effects characteristic of 1,4-benzodiazepines. The molecule has one chiral centre. Tofisopam was marketed in the racemate form and it was orally administered at 300 mg/day for its anxiolytic properties (Bernard et al. 2008).
The clinical success of tofisopam initiated further chemical and pharmacological investigations resulting in structurally related derivatives (Láng et al. 1985). Among them, the most active compounds show beside their tranquilizing (anti-aggressive) character particular pharmacological properties reminiscent of both anxiolytics and atypical neuroleptics (Andrási et al. 1987; Horváth et al. 1989).
The 2,3-BZDs tofisopam, nerisopam and girisopam were shown to specifically bind to a binding site in the striatum and related brain regions (Horváth et al. 1994). This binding site was identified as girisopam binding site (Horváth et al. 2000). The molecular correlate of that binding site could however not be identified to date. Compounds with affinity to the binding site were found to be active in models of anxiety including elevated maze, lick conflict test, light–dark box, and anti-aggressive behavior in fighting mice and in models of psychosis, i.e., in the apomorphine climbing test, the conditioned avoidance reaction and—only at high doses—in the catalepsy test. The pattern of distribution of this binding site largely overlaps with the distribution of the PDE-10 isoenzyme (Seeger et al. 2003). PDE-10 was recently shown to be a very interesting target for antipsychotic activity and drug development programs are ongoing to identify novel PDE-10 inhibitors (Schmidt et al. 2008).
We now show that tofisopam acts as an inhibitor of phosphodiesterases with some selectivity for PDE 2, 3, 4 and 10 and affinities in the range of 0.42–2.1 μM. Activity of tofisopam as PDE inhibitor was reported previously, supporting the findings of our study for PDE1 to PDE5 (Bernard et al. 2008). Recently, it could be also shown that tofisopam blocks PDE-10A isoenzymes with an affinity of 264 nM for the racemate without stereo-selectivity (Nielsen et al. 2007). The same group reported affinity to the PDE4D isoenzymes with affinity of S(−)-tofisopam of 117 nM and R(+)-tofisopam of 1,257 nM.
Phosphodiesterases are widely distributed throughout the body, and they serve diverse functions. While PDE-3 is known to selectively affect cardiac contractility by increasing the intracellular level of cyclic adenosine monophosphate (Goenen 1989), the other three phosphodiesterases targeted by tofisopam have potent CNS activity, and more specifically are discussed to be useful for amelioration of symptoms of psychosis. The oldest phosphodiesterase target for treatment of symptoms of psychosis is the PDE-4 isoenzyme. Rolipram, being the prototype PDE-4 inhibitor, was shown to have not only antidepressant and immune modulator, but also antipsychotic and cognitive enhancing activity (Kanes et al. 2007). However, the clinical utility of rolipram and other PDE-4 inhibitors is hampered by the fact that these compounds induce emesis, nausea and gastrointestinal side effect. Roflumilast is the only selective potent PDE4 inhibitor which is submitted for marketing approval for the treatment of chronic obstructive pulmonary disease, albeit with a narrow margin of safety (Field 2008). Roflumilast, however, is not published to be active in behavioral models of CNS diseases and it is not known whether roflumilast can penetrate the brain. The compound has a plasma protein binding of 98.9% (Bethke et al. 2007).
The discovery that PDE-10 is selectively expressed in medium spiny neurons of the striatum and the correlation of PDE-10 function with dopaminergic neurotransmission has placed PDE-10 as a highly interesting novel target for treatment of psychosis (Siuciak et al. 2006). PDE-10 inhibitors exhibit a broad spectrum of antipsychotic activity in different animal models (Siuciak et al. 2006; Siuciak 2008; Schmidt et al. 2008). Both, effects in models of positive and negative symptoms are reported. The pharmacological activity could be correlated not only to an inhibition of the D2-receptor-dominated pathways, but also to a facilitation of the D1-receptor-dominated prefrontal pathways. This combination of activity is believed to be highly interesting for the amelioration of negative symptoms of psychosis (Siuciak 2008). The target is subject to drug discovery programs, but none of these programs has reached a state of proof of efficacy in patients (Verhoest et al. 2009).
The PDE-2 isoenzyme is a further candidate for the treatment of symptoms of psychosis, however, with focus on the memory impairment contributing to the negative symptoms of psychosis (Boess et al. 2004; Domek-Łopacińska and Strosznajder 2008). Furthermore, anxiolytic effects may be mediated by PDE-2 inhibition (Masood et al. 2009).
We now show that tofisopam acts as a mixed blocker of all three psychosis treatment related targets. The affinity of tofisopam to these isoenzymes is in the same range or even higher than the affinity of rolipram which was found to have an affinity of 1.1 μM (Bernard et al. 2008). While tofisopam remains to be a low-affinity PDE inhibitor if compared with modern PDE inhibitors, it is possible that the pharmacological activity is mediated by its interaction with some or all of these phosphodiesterase isoenzymes. In volunteers administered a single dose of 100 mg tofisopam, a maximal plasma level of 328.8 ng/ml was reached, equal to about 0.9 μM. The apparent volume of distribution ranged from 523.8 to 5,154.1 l−1 indicating that tofisopam has higher concentration in extravascular tissue than in the vascular compartment. The half-life was found to be in the range of 4 h, indicating that upon three times daily administration of 100 mg some accumulation can be expected (Tóth et al. 2006). These data indicate that the steady-state tissue concentration can be expected to be in the range of or even higher than the IC50 of PDE-4, PDE-10, and potentially also PDE-2 resulting in a partial block of these enzymes.
In the in vivo model, the repeated administration of dizocilpine lead to the expected prolongation of immobility in the mice tested. It should be noted that the reactivity of the mice cannot be correlated to an acute drug-effect of dizocilpine, which is known to induce hyperactivity if administered to animals. Due to the washout time of 3 days after the last dose and in view of the half-life of dizocilpine in rodents in the range of 2 h (Vezzani et al. 1989), the behavioral modification of the mice may rather be correlated to a chronic plasticity. Our data indicate that the model initially described using phencyclidine can be generated using dizocilpine instead (Noda et al. 1995).
Tofisopam, administered twice daily to the mice, was capable of reducing the increased immobility in our model. The effect was evident 3 days after the last dose of tofisopam, indicating that the effect cannot be related to an acute effect of tofisopam on mouse behavior. The enhancement of immobility induced by subchronic administration of NMDA antagonists is attenuated by clozapine and risperidone, which are known to improve negative symptoms in patients, but not by haloperidol, which does not improve them (Noda et al. 1995, 2000). Tofisopam thus bears the potential to ameliorate negative symptoms of psychosis such as avolition; however, no complete normalization could be obtained. This may be related to the short half-life of tofisopam in rodents, but further experiments are needed to evaluate the potential of tofisopam or other (more potent) 2,3-BZDs in psychosis. Other models of negative symptoms of psychosis should be also employed to evaluate whether negative symptoms beyond avolition are also modified.
It may be discussed which phosphodiesterase isoenzymes may be responsible for the observed pharmacological action in mice. Both, PDE-4 and PDE-10 isoenzymes are involved in pharmacological action which may result in effects on negative symptoms of psychosis. Siuciak reviewed in 2008 the role of phosphodiesterases in psychosis and she concludes that both isoenzymes bear great potential for the treatment of positive symptoms, negative symptoms and/or cognitive deficits associated with schizophrenia (Siuciak 2008). Drugs targeting both targets may be of even higher interest. While the potential of such dual inhibitors has not yet been explored systematically and while no CNS-active drugs with selectivity for PDE-4 or PDE-10 are marketed so far, the availability of tofisopam enables a first insight in the potential of such dual inhibitors. Furthermore, the additional inhibition of PDE-2 may also contribute to the overall activity, adding anxiolytic effects and potentially also positive effects on cognitive function. Tofisopam is not afflicted with the well-known side effects of selective PDE-4 inhibitors. While Bernard et al. (2008) discuss that tofisopam may represent a novel structural class of tolerated PDE-4 inhibitors without emetogenic potential, an alternative explanation to the good tolerability may be that the combination of a partial block of multiple PDEs with additive or even synergistic pharmacological effects results in the observed pharmacology, while preventing the toxicity known from highly selective and potent compounds, dosed to induce a complete block of individual enzymes.
Other 2,3-BZDs may be systematically evaluated for their interaction with PDE isoenzymes and compounds with combined affinity to PDE-4 and PDE-10, but also to PDE-2 may be tested in respective animal models, with focus on dual or even triple inhibitors.
References
Abdel-Naby SM, Noda Y, Mahmoud HM, Mamiya T, Nagai T, Furukawa H, Nabeshima T (2001) Enhancement of immobility induced by repeated phencyclidine injection: association with c-Fos protein in the mouse brain. Behav Brain Res 124:71–76
Andrási F, Horváth K, Sineger E, Berzsenyi P, Borsy J, Kenessey A, Tarr M, Láng T, Kőrösi J, Hámori T (1987) Neuropharmacology of a new psychotropic 2,3-benzodiazepine. Arzneimittelforschung 37:1119–1124
Arnt J, Skarsfeldt T (1998) Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 18:63–101
Bernard P, Dufresne-Favetta C, Favetta P, Do QT, Himbert F, Zubrzycki S, Scior T, Lugnier C (2008) Application of drug repositioning strategy to TOFISOPAM. Curr Med Chem 15:3196–3203
Bethke TD, Bohmer GM, Hermann R, Hauns B, Fux R, Morike K, David M, Knoerzer D, Wurst W, Gleiter CH (2007) Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol 47:26–36
Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G (2004) Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 47:1081–1092
Domek-Łopacińska K, Strosznajder JB (2008) The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging. Brain Res 1216:68–77
Field SK (2008) Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin Investig Drugs 17:811–818
Goenen M (1989) Historical perspectives and update of amrinone. J Cardiothorac Anesth 3:15–23
Horváth K, Andrási F, Berzsenyi P, Pátfalusi M, Patthy M, Szabó G, Sebestyén L, Bagdy E, Kőrösi J, Botka P, Hámori T, Láng T (1989) A new psychoactive 5H-2,3-benzodiazepine with a unique spectrum of activity. Arzneimittelforschung 39:894–899
Horváth EJ, Palkovits M, Lenkei Z, Gyüre KI, Fekete MI, Arányi P (1994) Autoradiographic localization and quantitative determination of specific binding sites of anxiolytic homophthalazines (formerly called 2,3-benzodiazepines) in the striato-pallido-nigral system of rats. Brain Res Mol Brain Res 22:211–218
Horváth EJ, Horváth K, Hámori T, Fekete MI, Sólyom S, Palkovits M (2000) Anxiolytic 2,3-benzodiazepines, their specific binding to the basal ganglia. Prog Neurobiol 60:309–342
Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP (2007) Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience 144:239–246
Klebovich I, Abermann M (1993) Pharmacokinetics and metabolism of tofizopam (Grandaxin). Acta Pharm Hung 63:83–90
Láng T, Kőrösi J, Zólyomi G, Hámori T, Botka P (1985) Design and synthesis of 5H-2,3-benzodiazepines. In: Vizi ES, Furst S, Zsilla G (eds) Proc. 4th Cong. Hung. Pharmacol. Soc., Pergamon Press and Publishing House of the Hungarian Academy of Sciences, Oxford and Budapest, pp 91–97
MacKenzie S, Hastings S, Wells C (2010) Cyclic nucleotide phosphodiesterase assay technology. Curr Protoc Pharmacol 49:3.12.1–3.12.26
Masood A, Huang Y, Hajjhussein H, Xiao L, Li H, Wang W, Hamza A, Zhan CG, O’Donnell JM (2009) Anxiolytic effects of phosphodiesterase-2 inhibitors associated with increased cGMP signaling. J Pharmacol Exp Ther 331:690–699
Nielsen EB, Kehler J, Nielsen J, Brøsen P (2007) Patent: use of tofisopam as a PDE10A inhibitor. WO 2007/082546 A1
Noda Y, Yamada K, Furukawa H, Nabeshima T (1995) Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol 116:2531–2537
Noda Y, Kamei H, Mamiya T, Furukawa H, Nabeshima T (2000) Repeated phencyclidine treatment induces negative symptom-like behavior in forced swimming test in mice: imbalance of prefrontal serotonergic and dopaminergic functions. Neuropsychopharmacology 23:375–387
Petocz L (1993) Pharmacologic effects of tofizopam (Grandaxin). Acta Pharm Hung 63:79–82
Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, Hoffman WE, Lebel LA, McCarthy SA, Nelson FR, Proulx-LaFrance C, Majchrzak MJ, Ramirez AD, Schmidt K, Seymour PA, Siuciak JA, Tingley FD III, Williams RD, Verhoest PR, Menniti FS (2008) Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther 325:681–690
Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA, Varghese AH, Williams RD, Wylie PG, Menniti FS (2003) Immunohistochemical localization of PDE10A in the rat brain. Brain Res 985:113–126
Siuciak JA (2008) The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs 22:983–993
Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, Shrikhande A, Wong S, Menniti FS, Schmidt CJ (2006) Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology 51:386–396
Szegő J, Somogyi M, Papp E (1993) Excerpts from the clinical-pharmacologic and clinical studies of Grandaxin. Acta Pharm Hung 63:91–98
Tóth M, Bereczki A, Drabant S, Nemes KB, Varga B, Grézal G, Tömlő J, Lakner G, Klebovich I (2006) Gas chromatography nitrogen phosphorous detection (GC-NPD) assay of tofisopam in human plasma for pharmacokinetic evaluation. J Pharm Biomed Anal 41:1354–1359
Verhoest PR, Chapin DS, Corman M, Fonseca K, Harms JF, Hou X, Marr ES, Menniti FS, Nelson F, O’Connor R, Pandit J, Proulx-LaFrance C, Schmidt AW, Schmidt CJ, Suiciak JA, Liras S (2009) Discovery of a novel class of phosphodiesterase 10A inhibitors and identification of clinical candidate 2-[4-(1-methyl-4-pyridin-4-yl-1H-pyrazol-3-yl)-phenoxymethyl]-quinoline (PF-2545920) for the treatment of schizophrenia. J Med Chem 52:5188–5196
Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R (1989) Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther 249:278–283
Acknowledgments
The authors wish to thank EGIS Pharmaceuticals PLC (Budapest, Hungary) for a generous gift of tofisopam.
Conflict of interest
The authors declare they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rundfeldt, C., Socała, K. & Wlaź, P. The atypical anxiolytic drug, tofisopam, selectively blocks phosphodiesterase isoenzymes and is active in the mouse model of negative symptoms of psychosis. J Neural Transm 117, 1319–1325 (2010). https://doi.org/10.1007/s00702-010-0507-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0507-3