Abstract
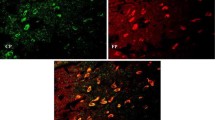
The staging of Lewy-related pathology in sporadic Parkinson’s disease (PD) reveals that many brain nuclei are affected in PD during different stages, except the ventral tegmental area (VTA), which is close related to the substantia nigra (SN) and enriched in dopamine (DA) neurons. Why DA neurons are selectively degenerated in the SN of PD is far from known. In the present study, we observed that the number of tyrosine hydroxylase immunoreactive neurons decreased and iron-staining positive cells increased in the SN, but not in the VTA, in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated PD mice. Increased expression of divalent metal transporter 1 and decreased expression of ferroportin 1 might associate with this increased nigral iron levels. Lipofuscin granular aggregations and upregulation of alpha-synuclein (α-synuclein) were also observed only in the SN. These results suggest that increased iron levels associate with the selective degeneration of DA neurons in the SN. The intracellular regulation mechanisms for the iron transporters may be different in the SN and VTA under the same conditions. Moreover, the lipofuscin granular aggregations and upregulation of α-synuclein were also involved in the selective degeneration of dopaminergic neurons in the SN.







Similar content being viewed by others
References
Bartzokis G, Cummings JL, Markham CH, Marmarelis PZ, Treciokas TA, Tishler TA, Marder SR, Mintz J (1999) MRI evaluation of brain iron in earlier- and later-onset Parkinson’s disease and normal subjects. Magn Reson Imaging 17:213–222
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45:182–184
Berg D, Siefker C, Ruprecht-Dörfler P, Becker G (2001) Echo pattern of substantia nigra and its relevance for motor function and motility in elderly subjects. Neurology 56:13–17
Braak H, Del Tredici K (2008) Invited Article: nervous system pathology in sporadic Parkinson disease. Neurology 70:1916–1925
Braak E, Sandmann-Keil D, Rub U et al (2001) Alpha-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol 101:195–201
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Cole NB, Murphy DD, Lebowitz J, Di Noto L, Levine RL, Nussbaum RL (2005) Metal-catalyzed oxidation of alpha-synuclein: helping to define the relationship between oligomers, protofibrils, and filaments. J Biol Chem 280:9678–9690
Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4:1318–1320
Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT Jr (2000) Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann N Y Acad Sci 920:42–45
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8):1437–1448
Ebadi M (2001) Introduction. Oxidative stress in mitochondria disorders of aging. Biol Signals Recept 10:5–13
Forno LS, DeLanney LE, Irwin I, Langston JW (1996) Electron microscopy of Lewy bodies in the amygdala-parahippocampal region: comparison with inclusion bodies in the MPTP-treated squirrel monkey. Adv Neurol 69:217–228
Friedlich AL, Tanzi RE, Rogers JT (2007) The 5′-untranslated region of Parkinson’s disease alpha-synuclein messengerRNA contains a predicted iron responsive element. Mol Psychiatry 12:222–223
German DC, Manaye K, Smith WK, Woodward DJ, Saper CB (1989) Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol 26:507–514
Giasson BI, Uryu K, Trojanowski JQ, Lee VM (1999) Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274:7619–7622
Golts N, Snyder H, Frasier M, Theisler C, Choi P, Wolozin B (2002) Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J Biol Chem 277:16116–16123
Gorell JM, Ordidge RJ, Brown GG, Deniau JC, Buderer NM, Helpern JA (1995) Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 45:1138–1143
Grant RJ, Clarke PB (2002) Susceptibility of ascending dopamine projections to 6-hydroxydopamine in rats: effect of hypothermia. Neuroscience 115:1281–1294
Hashimoto M, Hsu LJ, Sisk A, Xia Y, Takeda A, Sundsmo M, Masliah E (1998) Human recombinant NACP/alpha-synuclein is aggregated and fibrillated in vitro: relevance for Lewy body disease. Brain Res 799:301–306
He Y, Thong PS, Lee T, Leong SK, Shi CY, Wong PT, Yuan SY, Watt F (1996) Increased iron in the substantia nigra of 6-OHDA induced parkinsonian rats: a nuclear microscopy study. Brain Res 735:149–153
He Y, Lee T, Leong SK (1999) Time course of dopaminergic cell death and changes in iron, ferritin and transferrin levels in the rat substantia nigra after 6-hydroxydopamine (6-OHDA) lesioning. Free Radic Res 31:103–112
Hung HC, Lee EH (1996) The mesolimbic dopaminergic pathway is more resistant than the nigrostriatal dopaminergic pathway to MPTP and MPP + toxicity: role of BDNF gene expression. Brain Res Mol Brain Res 41:14–26
Hung HC, Lee EH (1998) MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med 24:76–84
Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, Stachelberger H, Youdim MB, Ben-Shachar D (1993) Iron and ferritin in substantia nigra in Parkinson’s disease. Adv Neurol 60:267–272
Jiang H, Qian ZM, Xie JX (2003) Increased DMT1 expression and iron content in MPTP-treated C57BL/6 mice. Sheng Li Xue Bao 55:571–576
Jiang H, Luan Z, Wang J, Xie J (2006) Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron-overload. Neurochem Int 49:605–609
Jiang H, Song N, Xu H, Zhang S, Wang J, Xie J (2010) Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res 20:345–356
Kostka M, Hogen T, Danzer KM et al (2008) Single particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J Biol Chem 283:10992–11003
Maingay M, Romero-Ramos M, Carta M, Kirik D (2006) Ventral tegmental area dopamine neurons are resistant to human mutant alpha-synuclein overexpression. Neurobiol Dis 23:522–532
McNaught KS, Perl DP, Brownell AL, Olanow CW (2004) Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Ann Neurol 56:149–162
Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC Jr, Lau YS (2002) Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res 956:156–165
Mochizuki H, Imai H, Endo K, Yokomizo K, Murata Y, Hattori N, Mizuno Y (1994) Iron accumulation in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced hemiparkinsonian monkeys. Neurosci Lett 168:251–253
Moore RY, Bloom FE (1978) Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci 1:129–169
Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B (2000) The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20:6048–6054
Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK (2007) Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J Neurosci 27:6914–6922
Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS (2001) Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 106:589–601
Ryvlin P, Broussolle E, Piollet H, Viallet F, Khafallah Y, Chazot G (1995) Magnetic resonance imaging evidence of decreased putaminal iron content in idiopathic Parkinson’s disease. Arch Neurol 52:583–588
Salazar J, Mena N, Hunot S, Prigent A et al (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci USA 105:18578–18583
Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB (1991) Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem 56:978–982
Song N, Jiang H, Wang J, Xie JX (2007) Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci Res 85:3118–3126
Sundstrom E, Fredriksson A, Archer T (1990) Chronic neurochemical and behavioral changes in MPTP-lesioned C57BL/6 mice: a model for Parkinson’s disease. Brain Res 528:181–188
Temlett JA, Landsberg JP, Watt F, Grime GW (1994) Increased iron in the substantia nigra compacta of the MPTP-lesioned hemiparkinsonian African green monkey: evidence from proton microprobe elemental microanalysis. J Neurochem 62:134–146
Wang J, Jiang H, Xie JX (2003) Correlation between iron levels and degeneration of dopaminergic neurons in rat nigrostriatal system during the early 6-OHDA lesions in medial forebrain bundle. Sheng Li Xue Bao 55:422–427
Wang J, Qian ZM, Jiang H, Xie J, Ke Y (2005) Treatment with nerve growth factor decreases expression of divalent metal transporter 1 and transferrin receptor in PC12 cells. Neurochem Int 47:514–517
Wang J, Xu HM, Yang HD, Du XX, Jiang H, Xie JX (2009) Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem Int 54:43–48
Waters CM, Hunt SP, Jenner P, Marsden CD (1987) An immunohistochemical study of the acute and long-term effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the marmoset. Neuroscience 23:1025–1039
Xu H, Jiang H, Wang J, Xie J (2010) Rg1 protects the MPP(+)-treated MES23.5 cells via attenuating DMT1 up-regulation and cellular iron uptake. Neuropharmacology 58(2):488–494
Zhang S, Wang J, Song N, Xie J, Jiang H (2009) Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol Aging 30:1466–1476
Acknowledgments
This study was supported by grants from the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2006CB500704), the National Foundation of Natural Science of China (30930036, 30870858) and the Natural Science Fund of Shandong Province for Distinguished Young Scholars (JQ200807).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, Z., Jiang, H., Xu, H. et al. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson’s disease. J Neural Transm 118, 361–369 (2011). https://doi.org/10.1007/s00702-010-0434-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0434-3