Summary.
In this review, we discuss the contribution of functional neuroimaging to the understanding of the cerebral control of gait in humans, both in healthy subjects and in patients with Parkinson’s disease. We illustrate different approaches that have been used to address this issue, ranging from the imaging of actual gait performance to the study of initiation and imagery of gait. We also consider related approaches focused on specific aspects of gait, like those addressed by repetitive foot movements. We provide a critical discussion of advantages and disadvantages of each approach, emphasizing crucial issues to be addressed for a better understanding of the neural control of human gait.
Similar content being viewed by others
Introduction
Most of the existing knowledge about the cerebral control of gait in mammals comes from studies in cats and rodents (Armstrong 1986; Drew et al. 1996; Rossignol et al. 2006). This work indicates that gait is regulated by cortical and subcortical structures, in particular during locomotion that requires accurate visuomotor coordination. However, it is unclear to what extent these findings can be extended to the voluntary control of human gait. Here we review recent developments on the cerebral bases of gait in humans, developments made possible by using different non-invasive neuroimaging techniques, both in healthy subjects and in clinical populations with gait disturbances.
Neuroimaging of gait is not straightforward. For instance, structural neuroimaging of patients with gait disorders has provided limited insights, given that cerebral lesions causing higher-level gait disorders are typically multiple, or diffuse (Masdeu 2001). In addition, functional neuroimaging poses practical problems, since techniques like positron emission tomography (PET), functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) require that subjects do not move their head during the sampling of task-related cerebral activity. These problems have been overcome by developing alternative neuroimaging techniques that allow for recording of cerebral activity during actual gait; by recording cerebral activity during motor planning of walking prior to walking initiation; by using tasks that share some cerebral processes with gait, without the need to engage in actual gait (like motor imagery of gait, or repetitive foot movements); and by recording cerebral activity in patients with gait disorders during rest.
We will discuss how functional neuroimaging has contributed to the understanding of human gait control, in both healthy subjects and — as an example of a common neurodegenerative disease characterized by gait difficulties — patients with Parkinson’s disease. We describe the different approaches that have been used and the methodological issues involved in these different approaches. Our goal is to provide a critical review of the available literature, and to emphasize that simply listing the various cortical and subcortical structures associated with the neural control of gait (for a recent related review see Hanakawa (2006)) will likely remain a sterile enterprise if these structures are not understood at the system level, i.e. by means of explicit and formal computational models (Grillner 2006).
Functional neuroimaging of gait in healthy subjects
Gait performance
An obvious approach that has been used to examine the neural control of gait in healthy subjects is to record cerebral activity during physical gait performance. An advantage of this approach is that cerebral activity is directly related to actual walking. However, there are also considerable disadvantages: studying a walking person does not allow for discriminating whether the evoked activity is due to sensory input or motor output, and only a limited number of neuroimaging techniques can be used (because of movement artefacts).
A few neuroimaging techniques are available that can assess cerebral activity during actual gait. To date, these involve nuclear neuroimaging techniques, near-infrared spectroscopy (NIRS) and transcranial magnetic stimulation (TMS — see Table 1). Nuclear neuroimaging techniques allow for recording of cerebral activity during gait by separating in time task performance from image acquisition. For example, single photon emission tomography with technetium-99m-hexamethyl-propyleneamine oxime ([99m[Tc]HM-PAO SPECT) records cerebral activity during walking by injecting radioactively labeled HM-PAO during locomotion, and recording cerebral activity afterwards with SPECT. When radioactively labeled HM-PAO is injected intravenously during gait, it is rapidly distributed in the brain in proportion to regional cerebral blood flow and retained in the brain for hours. Therefore, the distribution of HM-PAO at the time of scanning reflects the pattern of cerebral perfusion at the time of injection. Fukuyama et al. (1997) used this approach to map cerebral activity during walking, and showed that during gait cerebral activity increased in the supplementary motor area (SMA), medial primary sensorimotor area, striatum, cerebellar vermis and visual cortex. This was the first study to show changes in cortical activity during walking in human subjects. A later study by the same group demonstrated that the cerebral activity during walking is not only observed in cortical and subcortical structures, but also in the dorsal brainstem (Hanakawa et al. 1999b). This finding is important, because it is one of the few observations suggesting the presence of brainstem locomotor centres in humans. However, given the limitations of SPECT, gait-related cerebral activity could only be compared with a physical rest condition (lying down with eyes open) in both studies. This raises the issue of whether those cerebral changes are specifically related to gait, over and above changes in somatosensory feedback from the lower limbs between gait and rest.
Another technique that allows for recording of cerebral activity during actual gait is NIRS. NIRS records the transmission and absorption of NIR light by human tissue. The skull does not absorb much infrared light, and therefore NIRS can be used to measure the levels of oxygenated, deoxygenated and total hemoglobin related to neural activity in superficial cortical areas. The optodes of NIR systems are fixed to the skull, and therefore head movements are allowed during measurement. Cerebral activity can be assessed while subjects are walking on a treadmill. NIRS has several advantages compared to HM-PAO SPECT: it does not involve a radio-active tracer, it has a better temporal resolution, and it allows for comparing several conditions. For instance, using NIRS, Miyai et al. (2001) were able to compare cerebral activities evoked during gait, alternating foot movements, arm swing, and motor imagery of gait. Gait-related responses along the central sulcus were medial and caudal to activity associated with arm swing, in agreement with the known somatotopic organization of the motor cortex. Crucially, these authors showed that walking increased cerebral activity bilaterally in the medial primary sensori-motor cortices and the SMA, and to a greater extent than the alternation of foot movements. Unfortunately, the spatial distribution and intensity of these responses were not statistically compared. In a different NIRS study, Suzuki et al. (2004) examined the effect of different walking speeds on cerebral activity. They demonstrated that cerebral activity in the prefrontal cortex and premotor cortex tended to increase as locomotor speed increased, whereas cerebral activity in the medial sensori-motor cortex was not influenced by locomotor speed. In summary, NIRS has been particularly useful for studying the cortical bases of locomotor control. Unfortunately, given the limited penetration of infrared light (a few centimeters from the skull surface), this technique can only assess the responses of the most superficial portions of the cerebral cortex.
A third technique that has been used to examine the neural substrate of gait during actual walking is TMS. TMS is a noninvasive method to excite neurons in the brain. If TMS is applied to the primary motor cortex, it can evoke muscle activity which can be recorded using electromyography. TMS has been useful to examine the contribution of the corticospinal tract to the control of gait (see e.g. (Schubert et al. 1997; Christensen et al. 1999; Petersen et al. 2001; Camus et al. 2006). However, given that the use of this technique has been circumscribed to the corticospinal tract, we will not discuss it at length.
Gait initiation
Recording of EEG during walking is challenging due to movement artefacts. However, some authors have been able to record electrical activity with scalp electrodes prior to or during gait initiation (Yazawa et al. 1997; do Nascimento et al. 2005). This approach has great advantages, since it provides a direct measure of electrophysiological activity in the brain at high temporal resolution. In addition, there are only minimal confounds of changes in sensory input, because the recording is performed prior to the onset of movement. For instance, Yazawa et al. (1997) examined EEG in a period immediately preceding gait initiation, when subjects had just received an auditory stimulus and were waiting for a second auditory stimulus in response to which they were asked to initiate gait as quickly as possible. They found stronger event-related potentials (contigent negative variation) in the medial central region (Cz) when comparing EEG activity preceding externally-cued gait initiation with activity preceding foot dorsiflexion. This EEG difference indicates that the medial frontal cortex, over and above its role in initiating a simple foot movement, supports the initiation of gait, presumably through the synchronized activity of a large number of dendritic trees (Luck 2005). However, given the low spatial resolution of this technique, it remains to be seen from which portion of the medial frontal cortex this activity is generated from.
Motor imagery of gait
Given the plethora of technical problems and limitations associated with assessing the cerebral bases of true gait control, several research groups have chosen to focus their efforts on the more tractable aspects. Accordingly, some studies have investigated motor imagery of gait, i.e. the mental simulation of gait without actual execution (Miyai et al. 2001; Malouin et al. 2003; Jahn et al. 2004; Sacco et al. 2006). This approach exploits the documented neural and cognitive overlap between movement planning and motor imagery: imagining a movement relies on neural processes similar to those evoked during actual performance of the same movement (Lang et al. 1994; Stephan et al. 1995; Porro et al. 1996; Roth et al. 1996; Deiber et al. 1998). Although most of the evidence for this cerebral overlap between simulation and execution of a movement has been obtained from finger and hand movements, Miyai et al. (2001) combined fMRI and NIRS measurements to show a degree of overlap between actual and imagined gait. Using motor imagery to study the cerebral correlates of gait provides considerable practical advantages, since motor imagery does not involve any actual movements, and it can be studied in a recumbent position compatible with techniques like fMRI and PET. This is important, since these techniques provide relatively high spatial resolution and whole-brain coverage. Furthermore, there are also conceptual advantages in using motor imagery. It has been argued that the internal simulation of an action, as evoked during motor imagery, constitutes the core element of a motor plan (Jeannerod 1994). Furthermore, motor imagery allows one to study cognitive and cerebral properties of movement representations independently from motor output and sensory feedback (de Lange et al. 2005). However, the latter is true insofar subjects are genuinely involved in a motor simulation, i.e. they imagine themselves moving, rather than using visual imagery of someone else moving, or visual imagery of the visuospatial processes involved in walking. Given the fact that gait is a learned automatic movement, it might be particularly difficult to voluntarily imagine the movements involved in walking. For instance, when subjects are trained to focus their attention on the movements of their legs by means of basic tango lessons and motor imagery of the performed steps, there is an expansion of activity in the bilateral motor areas, and a reduction of visuospatial activation in the right posterior cerebral cortex during motor imagery of gait performed after this training compared to before the training (Sacco et al. 2006). These findings suggest that focusing subject’s attention on the movements involved in walking decreases the role of visual imagery processes in favor of motorkinesthetic ones. Therefore, when using motor imagery to study gait, it becomes particularly important to use tasks that allows for monitoring subjects’ performance and prove their engagement in first person motor imagery (Jeannerod 1994).
Motor imagery of gait has been examined using NIRS fMRI and H2 15O-PET (see Table 1). Malouin et al. (2003) used H2 15O-PET to compare cerebral activity evoked during motor imagery of standing, initiating gait, walking, and walking with obstacles. Prior to the experiment, subjects were shown a video of each imagery condition. The video was taken from a first person perspective to facilitate first person motor imagery and to standardize imagined walking speed. During PET scans, subjects were asked to imagine the different movements, from a first person perspective and with their eyes closed. Motor imagery of walking increased cerebral activity in the pre-SMA when compared to imagined standing, and in the left visual cortex and caudate nucleus when compared to imagery of gait initiation. Comparing motor imagery of walking with or without obstacles increased cerebral activity in the precuneus bilaterally, the left SMA, the right parietal inferior cortex and the left parahippocampal gyrus. These results are important, since they are the first to illustrate that the circuitry supporting gait can extend beyond core motor regions, and it can be modulated by the difficulty of the (imagined) locomotor task. Prior to scanning, imagery performance was monitored using a chronometry test and a questionnaire, which both suggested that subjects were able to perform motor imagery. However, during scanning imagery performance was monitored through its effect on heart rate, and this parameter cannot easily distinguish between the effects of performing visual or motor imagery. Therefore, although the chronometry test and questionnaire suggests that subjects were able to perform first person motor imagery prior to the experiment, it remains unclear whether the effects reported in this study are related to cognitive modulations of gait-related cerebral activity, or to inter-subject variations in imagery performance.
In another study, Jahn et al. (2004) compared cerebral activity during motor imagery of standing, walking and running using fMRI. To induce first person motor imagery and to standardize motor imagery performance, subjects were trained to perform the actual movements on a basement floor prior to the imagery experiment. During motor imagery, subjects closed their eyes and imagined performing the same movements from a first person perspective. However, there was no behavioral quantification of imagery performance. Cerebellar activation increased during motor imagery of running but not during motor imagery of walking and standing. Vestibular and somatosensory cortex were deactivated during running but not during walking. These findings suggest that speed of gait is under the control of a cerebellar locomotor centre, and that cortical processing of vestibular and somatosensory information is particularly important during walking. Unfortunately, the between-tasks differences in cerebral activity were not formally tested, thus no clear inference can be drawn about the involvement of these structures in the different locomotor tasks.
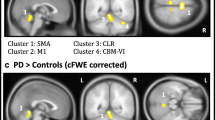
In our laboratory, we have also investigated the cerebral bases of gait control by using fMRI in conjunction with an imagery task (Bakker et al. 2006). We have built upon the studies described in this review, providing an objective quantification of first-person motor imagery during the fMRI scan. We have manipulated both the type of imagery evoked by the task settings (motor or visual imagery) and the task difficulty. Subjects were presented with photographs of walking trajectories taken from a first person perspective. The photographs showed a corridor with a white path (Fig. 1). The path could have two different widths (9 and 27 cm), and five different distances (2, 4, 6, 8 and 10 m). During the imagery of gait trials, the subjects were asked to imagine walking along the path. During a control visual imagery task, the subjects were asked to imagine seeing a small disk moving along the path. In both cases, subjects reported the onset and the offset of the imagined movement by pressing a button. This procedure allowed us to quantify imagery time (i.e. the time between the two button presses) on a trial-by-trial basis, and during the acquisition of the fMR images. The task allowed us to verify that the subjects were engaged in imagery. This could be done by testing whether imagined movement times increased as a function of walking trajectory length. Crucially, we could also verify that the subjects were specifically engaged in motor imagery of gait. This could be done by testing whether imagined movement times increased as a function of path width during the motor imagery trials, and not in the visual imagery trials. The assumption here is that motor imagery should be sensitive to the biomechanical constraints imposed by a narrow walking path that allows only for positioning one foot at a time on the path. We found that imagery time increased with increasing movement distance and decreasing path width (Bakker et al. 2006). During visual imagery trials, imagery time increased with distance, but not with path width. In other words, during imagery of gait only, there was an inverse and logarithmic relationship between movement difficulty and imagined movement time [Fitts’ law, (Fitts 1954)]. This result strongly suggests that the subjects (as a group) were using first person motor imagery. However, we cannot completely exclude that some subjects may have used explicit knowledge about the time it takes to execute the movements to solve the task. In cerebral terms, we found that activity increased bilaterally in the superior parietal lobule and in the right superior middle occipital gyrus during motor imagery of gait involving a narrow path (Fig. 1). This finding indicates that the increased spatial accuracy and sensorimotor integration required to walk along a narrow path is supported by cortical structures outside primary motor regions.
a) Examples of photographs of broad (left) or narrow (right) walking trajectories presented to the subjects during the motor imagery (MI) experiment. b) Statistical parametric map (p<0.05 family wise error (FWE) corrected for multiple comparisons) showing increased cerebral activity bilaterally in the superior parietal lobule and in the right superior middle occipital gyrus during MI along a narrow path (compared to MI along a broad path), superimposed on a rendered brain viewed from the top. c) Beta weights (mean ± sem) of the left superior parietal lobule (−16, −50, 64) for the broad and narrow path, separate for motor imagery and visual imagery (VI)
Repetitive leg or foot movements
A further approach to asses the cerebral bases of gait control is to examine repetitive leg or foot movements, as a surrogate for gait. The rationale is that these movements are thought to rely on partly similar neural processes as those used during walking. For example, alternating foot flexion-extension movements and bicycling movements require internal pacing and interlimb coordination, mechanisms that are also required during gait. Miyai et al. (2001) combined NIRS and fMRI and showed that foot-extension flexion movements indeed generate a similar brain activation pattern to that associated with walking. Using foot or leg movements to study the cerebral correlates of gait provides considerable practical advantages, since these movements can be performed with minimal movements of the head, and in a recumbent position. Another advantage is that voluntary foot or leg movements can be matched with similar passive movements, and this procedure allows one to dissociate responses to somatosensory input from the volitional aspects of the task (Mima et al. 1999). However, the relationship between lower limb movements and gait is limited. Leg or foot movements do not allow for examining the way in which several important features of gait (such as the upright stance, truncal control and co-innervation of gluteal and leg muscles) are controlled. Therefore, it remains to be established to what extent the motor control of repetitive foot or leg movements has cerebral analogies with the motor control of gait.
Repetitive foot movements have been investigated using NIRS, fMRI and H2 15O-PET (see Table 1). The group of Sahyoun used fMRI to compare active versus passive unilateral foot extension-flexion movements and found that during active compared to passive foot movements cerebral activity increased in the somatosensory cortex, SMA, cingulate motor area, secondary somatosensory cortex, insular cortices, putamen, thalamus and cerebellum (Sahyoun et al. 2004). This suggests that both cortical and subcortical structures are involved in the motor control of rhythmic foot movements. In another study, de Jong et al. (2002) examined cerebral activity during antiphase flexion and extension movements of the two upper and the two lower limbs using H2 15O-PET. They hypothesized that a common neural circuitry would be involved in antiphase movement, independently of whether they would be performed with the two upper or lower limbs. For both the arms and legs, cerebral activations related to antiphase movements were distributed over the right anterior parietal and right dorsal premotor cortex, suggesting that these structures support the sensorimotor integration required for antiphase movements. However, it is important to realize that controlling flexion-extensions of the foot is much simpler than controlling gait. For example, gait involves the coordination between a large number of body parts, and the integration of vestibular, visual, and somatosensory signals. A particularly delicate element of gait is the precise inter-limb timing (Plotnik et al. 2005), and this will be difficult to mimick using repetive foot movements, even when performed alternatingly in both feet.
The issue of inter-limb timing can perhaps be addressed by studying bicycle movements. Indeed, Christensen et al. (2000) used such bicycle movements to exactly matched active and passive movements, while addressing inter-limb coordinations. Using H2 15O-PET, they found that both passive and active bicycling increased cerebral activity bilaterally in primary sensorimotor cortices, SMA, and the anterior part of the cerebellum. When passive bicycling was subtracted from active bicycling, significant activation was found in the leg area of the primary motor cortex and the precuneus. These findings suggest that there is a significant cerebral involvement in the motor control of rhythmic motor tasks such as bicycling. However, given that it was quite difficult for subjects to remain absolutely relaxed during passive bicycle movement, some of the activity observed during the passive movements might be due to unwanted muscular activity. More importantly, it is unlikely that the cerebral network involved in the motor control of bicycling is confined to the primary motor cortex and precuneus.
Functional neuroimaging of gait in Parkinson’s disease
Insufficient knowledge of underlying gait mechanisms not only exists for healthy subjects, but also for patients with gait disturbances of a central neural origin, like patients with Parkinson’s disease (PD). Gait disturbance in PD is thought to originate, at least in part, from nigrostriatal dopamine deficiency, that in turn alters the basal gangliabrainstem circuits and the basal ganglia-thalamo-cortical systems (Bloem et al. 2004). The precise pathophysiological mechanism, however, needs to be elucidated.
Gait performance in PD
A first approach that has been used to examine the neural control of gait in patients with PD is to measure cerebral activity during gait performance and to compare it with that measured during gait performance of healthy subjects. An advantage of this approach is that cerebral activity is directly related to gait performance. However, this advantage should be weighted against the intricacies of matching both performance and task difficulty across patients and control groups.
Actual gait in PD has been investigated using HMPAO-SPECT and DAT-PET (see Table 2). The group of Hanakawa et al. (1999b) has described two studies using HM-PAO-SPECT comparing regional cerebral blood flow during treadmill walking. In the first study, they compared mildly to moderately impaired PD patients with agematched healthy controls, while walking at the same speed on a treadmill. The PD patients showed a relative decrease in brain activity in the left medial frontal lobe, right precuneus and left cerebellar hemisphere. In contrast there was an increased cerebral blood flow in the cerebellar vermis, right insula, left temporal cortex and left cingulate gyrus. The reduced brain perfusion in the frontal motor areas fits with similar results obtained during arm and finger movements (Berardelli et al. 2001), pointing to a systematic alteration of activity in this region during motor performance in PD patients. The overactivity in the cerebellar vermis in combination with an underactivity in the cerebellar hemisphere might be associated with a loss of lateral gravity shift in PD.
The same group performed a follow-up study, investigating the effects of visual cuing on gait in PD patients. It is known that visual cues can help PD patients to overcome gait disturbances, especially freezing (Bloem et al. 2004). Accordingly, Hanakawa et al. (1999a) applied transverse or parallel white lines to the walking surface of a treadmill. Gait abnormality in PD patients was ameliorated in the transverse line situation. The authors compared brain activity evoked during gait in the transverse line condition and in the parallel line condition. In both controls and PD patients, there were increased responses in the posterior parietal cortex and cerebellar hemispheres during gait in the transverse line condition. Crucially, the premotor area activation was significantly greater for PD patients than for controls, suggesting that this region may be involved in visuomotor control of gait in patients with a “paradoxical” (externally cued) gait. However, it remains unclear whether these results were influenced by residual difference in performance between the two groups. Despite the use of a treadmill, task performance was not completely matched between patients and controls. More generally, one might wonder whether cerebral activity associated with the forced locomotion evoked on a treadmill is comparable to that evoked during spontaneous gait, or to parkinsonian gait.
It might be argued that a meaningful approach to the cerebral correlates of gait disturbances in PD patients should directly consider the modulatory effects of dopaminergic activity in the basal ganglia on the motor system. Accordingly, Ouchi et al. investigated changes in dopamine transporter (DAT) availability during standing and during gait in unmedicated PD patients and in normal subjects (Ouchi et al. 2001). Subjects underwent two serial PET scans ([11C]CFT-PET). The second scan was performed after tracer injection and a subsequent walk of 50 min. Participants walked at their own pace along a white line in the corridor back and forth. Normal subjects were requested to walk more slowly. Stride length and cadence were not significantly different between PD patients and controls. The latter group showed a significant reduction of [11C]CFT uptake during gait in both the putamen and in the caudate. PD patients showed a similar reduction of [11C]CFT uptake in the caudate (and orbitofrontal cortex), but not in the putamen. This study points to alterations in the availability of DAT in the medial striatum as a source of pathophysiological changes in gait performance in PD patients. However, the specificity of these effects remains to be tested, given that changes in local regional blood flow during exercise may affect levels of tracer binding. Furthermore, PD patients showed difficulties while turning at each end of the corridor, suggesting that there might have been residual differences in task difficulty between patients and controls.
Gait initiation in PD
One study by the group of Vidailhet has investigated the initiation of gait in PD by means of the Bereitschaftspotential (BP) using EEG (Vidailhet et al. 1993). The BP is a movement-related potential, with two main components; an early one (BP1) lasting from about 1.2 to 0.5 sec before movement onset, and a late component (BP2) shortly (0.5 sec) before movement onset (Deecke et al. 1976). The BP is an electrical sign of participation of the SMA prior to volitional movement (Deecke and Kornhuber 1978). Vidailhet et al. found that PD patients showed little change in either early or late components of the BP between foot dorsiflexion and stepping tasks. In contrast, controls subjects showed a larger peak in the early BP phase before a stepping movement than preceding a voluntary dorsiflexion. Distribution of the BP was most conspicuous over midline scalp positions (Fz, Cz), but also ipsilaterally (P4, posterior parietal) to the foot movement (Vidailhet et al. 1993). The altered activity over medial frontal motor areas fits the imaging results obtained during actual gait (Hanakawa et al. 1999b), emphasizing that PD patients have altered motor planning activity well in advance of gait execution.
Baseline perfusion in PD
Under the assumption that pathological alterations in brain activity are likely to be present not only during task performance but also during rest, some authors have examined baseline cerebral perfusion to investigate the cerebral bases of altered neural correlates of gait control in PD patients. This approach has the considerable advantage of perfectly matched “performance” across different subject groups. However, when using this approach to compare gait problems between two different groups of subjects, it is important to closely match the different groups. In the ideal situation, the only difference between the different subject groups would be their gait problems, and this is difficult to achieve in practice where gait is closely related to other relevant variables such as disease severity and disease duration. In PD, baseline perfusion has been used to examine freezing problems in patients with Parkinson’s disease, and to examine the effects of gait training.
Freezing of gait is a unique and extremely debilitating symptom of PD, with an unknown pathophysiological mechanism. There have been a few studies that tried to localize the altered cerebral activity associated with freezing. Using 123I-IMP-SPECT, Matsui et al. (2005) compared cerebral activity in PD patients with and without freezing, at comparable clinical stages of the disease. SPECT scanning was performed in a supine condition, at rest. Perfusion of the orbitofrontal area (Brodmann area 11) in the freezing of gait group was decreased, as compared to the non-freezing group. Another study has addressed this issue, but Fabre et al. (1998), using 133Xenon-SPECT in patients with PD and severe “off” freezing, could not find any specific effect in the orbitofrontal area. Finally, Bartels et al. (2006) addressed this issue using better spatial resolution than the previous studies. 2-deoxy-2[18F]fluoro-D-glucose-PET (FDG-PET) and 18[F]-6-fluoro-levodopa (FDOPA)-PET was used to compare striatum decarboxylase activity in two groups of PD patients with and without freezing of gait. Lower putaminal FDOPA uptake with increased FDG uptake was observed in freezing PD patients, whereas caudate uptake of both FDG and FDOPA was reduced. Furthermore, in freezing patients a decreased FDG uptake was found in the parietal cortices. This last study, did not show differences in the frontal lobe. Taken together, these studies suggest that medial frontal and basal ganglia might be altered in PD patients with freezing of gait, but at the moment the consistency of the results prevents any clear inference.
Finally, imaging techniques have been recently used to assess the effect of a gait rehabilitation program in PD patients. del Olmo et al. (2006) recorded baseline cerebral perfusion with FDG-PET in PD patients that walked with or without rhythmic auditory cues. The measurements were made before and after 4 weeks of a rehabilitation program based on auditory cues, aimed at minimizing the temporal variability of gait. The rationale for using this program was that PD patients depend strongly on external cues, both visual and auditory, to initiate or maintain walking. Before therapy, PD patients showed a significant hypometabolism in the right parietal lobe, temporal lobes, and left frontal lobe. Hypermetabolism was found in the left cerebellum. After therapy a significant increment of metabolism was found in the right cerebellum, right parietal lobe and temporal lobes, combined with a decrement of temporal variability during gait. This study shows training-related changes both in behavior and cerebral glucose metabolism. However, it remains to be seen whether the cerebral changes are specifically related with clinical improvements across a large number of patients.
Conclusion
In this review we have illustrated how functional neuroimaging techniques have been used to obtain information about the cerebral bases of gait control in healthy subjects, and about gait disturbances in patients with PD. Taken together, these studies have shown that the medial aspect of the motor cortex, controlling the lower limbs, can be modulated by different portions of the motor system, from posterior parietal cortex to the basal ganglia and the cerebellum. The basal ganglia-thalamo-cortical system presumably plays a major role in gait disorders in patients with PD.
In the clinical domain, given that several aspects of gait disturbances seem to be related to motor planning and rhythmic pacing, rather than motor execution per se, it appears reasonable to use motor imagery of gait and repetitive foot movements in patients with disorders such as PD. Using these approaches might also open the possibility to use other techniques, like magnetoencephalography (MEG) and TMS, which provide direct measures of neural activity at high temporal resolution. More importantly, these techniques would allow one to investigate cerebral circuits supporting gait, rather than a collection of brain regions showing altered metabolism related to gait. The time seems ripe for assembling these scattered observations into a coherent computational model of gait control in humans, able to generate testable predictions. Up to now computational models on the neural control of gait have mainly focused on how the central pattern generators coordinate walking movements (Grillner 2006). We think it would be useful to start developing computational models that focus on how cortical and subcortical structures are involved in visuomotor control during walking. Such models have already been used to examine the cerebral circuits underlying visuomotor control of arm movements (see for example (Ghahramani and Wolpert 1997; Nakahara et al. 2001). The findings of the studies reviewed in this paper might serve as a basis for developing such computational models.
References
DM Armstrong (1986) ArticleTitleSupraspinal contributions to the initiation and control of locomotion in the cat Prog Neurobiol 26 273–361 10.1016/0301-0082(86)90021-3 Occurrence Handle10.1016/0301-0082(86)90021-3 Occurrence Handle1:STN:280:DyaL283ovVSrtw%3D%3D Occurrence Handle3526411
M Bakker BR Bloem FP de Lange I Toni (2006) ArticleTitleNeuroimaging of gait: an imagery approach Neuroimage 31 S150 10.1016/j.neuroimage.2006.04.133 Occurrence Handle10.1016/j.neuroimage.2006.04.133
AL Bartels BM de Jong N Giladi JD Schaafsma RP Maguire L Veenma J Pruim Y Balash MB Youdim KL Leenders (2006) ArticleTitleStriatal dopa and glucose metabolism in PD patients with freezing of gait Mov Disord 21 1326–1332 10.1002/mds.20952 Occurrence Handle10.1002/mds.20952 Occurrence Handle16721756
A Berardelli JC Rothwell PD Thompson M Hallett (2001) ArticleTitlePathophysiology of bradykinesia in Parkinson’s disease Brain 124 2131–2146 10.1093/brain/124.11.2131 Occurrence Handle10.1093/brain/124.11.2131 Occurrence Handle1:STN:280:DC%2BD3Mnnt1Onuw%3D%3D Occurrence Handle11673316
BR Bloem JM Hausdorff JE Visser N Giladi (2004) ArticleTitleFalls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena Mov Disord 19 871–884 10.1002/mds.20115 Occurrence Handle10.1002/mds.20115 Occurrence Handle15300651
M Camus J Pailhous M Bonnard (2006) ArticleTitleOn-line flexibility of the cognitive tuning of corticospinal excitability: a TMS study in human gait Brain Res 1076 144–149 10.1016/j.brainres.2005.12.001 Occurrence Handle10.1016/j.brainres.2005.12.001 Occurrence Handle1:CAS:528:DC%2BD28XisF2ntbo%3D Occurrence Handle16473341
LO Christensen P Johannsen T Sinkjaer N Petersen HS Pyndt JB Nielsen (2000) ArticleTitleCerebral activation during bicycle movements in man Exp Brain Res 135 66–72 10.1007/s002210000493 Occurrence Handle10.1007/s002210000493 Occurrence Handle1:STN:280:DC%2BD3M7js1yksA%3D%3D Occurrence Handle11104128
LO Christensen H Morita N Petersen J Nielsen (1999) ArticleTitleEvidence suggesting that a transcortical reflex pathway contributes to cutaneous reflexes in the tibialis anterior muscle during walking in man Exp Brain Res 124 59–68 10.1007/s002210050600 Occurrence Handle10.1007/s002210050600 Occurrence Handle1:STN:280:DyaK1M7isFyksg%3D%3D Occurrence Handle9928790
BM de Jong KL Leenders AM Paans (2002) ArticleTitleRight parieto-premotor activation related to limb-independent antiphase movement Cereb Cortex 12 1213–1217 10.1093/cercor/12.11.1213 Occurrence Handle10.1093/cercor/12.11.1213 Occurrence Handle1:STN:280:DC%2BD38nitF2hsw%3D%3D Occurrence Handle12379609
FP de Lange P Hagoort I Toni (2005) ArticleTitleNeural topography and content of movement representations J Cogn Neurosci 17 97–112 10.1162/0898929052880039 Occurrence Handle10.1162/0898929052880039 Occurrence Handle15701242
L Deecke B Grozinger HH Kornhuber (1976) ArticleTitleVoluntary finger movement in man: cerebral potentials and theory Biol Cybern 23 99–119 10.1007/BF00336013 Occurrence Handle10.1007/BF00336013 Occurrence Handle1:STN:280:DyaE283jt1CltA%3D%3D Occurrence Handle949512
L Deecke HH Kornhuber (1978) ArticleTitleAn electrical sign of participation of the mesial ‘supplementary’ motor cortex in human voluntary finger movement Brain Res 159 473–476 10.1016/0006-8993(78)90561-9 Occurrence Handle10.1016/0006-8993(78)90561-9 Occurrence Handle1:STN:280:DyaE1M%2FotlKmtg%3D%3D Occurrence Handle728816
MP Deiber V Ibanez M Honda N Sadato R Raman M Hallett (1998) ArticleTitleCerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography Neuroimage 7 73–85 10.1006/nimg.1997.0314 Occurrence Handle10.1006/nimg.1997.0314 Occurrence Handle1:STN:280:DyaK1c3hvFegtw%3D%3D Occurrence Handle9571132
MF del Olmo P Arias MC Furio MA Pozo J Cudeiro (2006) ArticleTitleEvaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients — a combined motor and [18F]-FDG PET study Parkinsonism Relat D 12 155–164 10.1016/j.parkreldis.2005.11.002 Occurrence Handle10.1016/j.parkreldis.2005.11.002
OF do Nascimento KD Nielsen M Voigt (2005) ArticleTitleInfluence of directional orientations during gait initiation and stepping on movement-related cortical potentials Behav Brain Res 161 141–154 10.1016/j.bbr.2005.02.031 Occurrence Handle10.1016/j.bbr.2005.02.031 Occurrence Handle15904721
T Drew W Jiang B Kably S Lavoie (1996) ArticleTitleRole of the motor cortex in the control of visually triggered gait modifications Can J Physiol Pharm 74 426–442 10.1139/cjpp-74-4-426 Occurrence Handle10.1139/cjpp-74-4-426 Occurrence Handle1:CAS:528:DyaK28XksV2ju7w%3D
N Fabre C Brefel U Sabatini P Celsis JL Montastruc F Chollet O Rascol (1998) ArticleTitleNormal frontal perfusion in patients with frozen gait Mov Disord 13 677–683 10.1002/mds.870130412 Occurrence Handle10.1002/mds.870130412 Occurrence Handle1:STN:280:DyaK1czkvFGlsQ%3D%3D Occurrence Handle9686774
PM Fitts (1954) ArticleTitleThe information capacity of the human motor system in controlling the amplitude of movement J Exp Psychol 47 381–391 10.1037/h0055392 Occurrence Handle10.1037/h0055392 Occurrence Handle1:STN:280:DyaG2c%2FpsVymtw%3D%3D Occurrence Handle13174710
H Fukuyama Y Ouchi S Matsuzaki Y Nagahama H Yamauchi M Ogawa J Kimura H Shibasaki (1997) ArticleTitleBrain functional activity during gait in normal subjects: a SPECT study Neurosci Lett 228 183–186 10.1016/S0304-3940(97)00381-9 Occurrence Handle10.1016/S0304-3940(97)00381-9 Occurrence Handle1:CAS:528:DyaK2sXks1Gntr8%3D Occurrence Handle9218638
Z Ghahramani DM Wolpert (1997) ArticleTitleModular decomposition in visuomotor learning Nature 386 392–395 10.1038/386392a0 Occurrence Handle10.1038/386392a0 Occurrence Handle1:CAS:528:DyaK2sXit1Wrsro%3D Occurrence Handle9121554
S Grillner (2006) ArticleTitleBiological pattern generation: the cellular and computational logic of networks in motion Neuron 52 751–766 10.1016/j.neuron.2006.11.008 Occurrence Handle10.1016/j.neuron.2006.11.008 Occurrence Handle1:CAS:528:DC%2BD28XhtlCgu7fI Occurrence Handle17145498
T Hanakawa (2006) ArticleTitleNeuroimaging of standing and walking: special emphasis on Parkinsonian gait Parkinsonism Relat D 12 S70–S75 10.1016/j.parkreldis.2006.05.009 Occurrence Handle10.1016/j.parkreldis.2006.05.009
T Hanakawa H Fukuyama Y Katsumi M Honda H Shibasaki (1999a) ArticleTitleEnhanced lateral premotor activity during paradoxical gait in Parkinson’s disease Ann Neurol 45 329–336 10.1002/1531-8249(199903)45:3<329::AID-ANA8>3.0.CO;2-S Occurrence Handle10.1002/1531-8249(199903)45:3<329::AID-ANA8>3.0.CO;2-S Occurrence Handle1:STN:280:DyaK1M7ntValsQ%3D%3D
T Hanakawa Y Katsumi H Fukuyama M Honda T Hayashi J Kimura H Shibasaki (1999b) ArticleTitleMechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study Brain 122 IssueIDPt 7 1271–1282 10.1093/brain/122.7.1271 Occurrence Handle10.1093/brain/122.7.1271
K Jahn A Deutschlander T Stephan M Strupp M Wiesmann T Brandt (2004) ArticleTitleBrain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging Neuroimage 22 1722–1731 10.1016/j.neuroimage.2004.05.017 Occurrence Handle10.1016/j.neuroimage.2004.05.017 Occurrence Handle15275928
M Jeannerod (1994) ArticleTitleThe representing brain: neural correlates of motor intention and imagery Behav Brain Sci 17 187–245 Occurrence Handle10.1017/S0140525X00034026
W Lang L Petit P Hollinger U Pietrzyk N Tzourio B Mazoyer A Berthoz (1994) ArticleTitleA positron emission tomography study of oculomotor imagery Neuroreport 5 921–924 10.1097/00001756-199404000-00017 Occurrence Handle10.1097/00001756-199404000-00017 Occurrence Handle1:STN:280:DyaK2czjvFCmsQ%3D%3D Occurrence Handle8061297
JL Luck (2005) An introduction to event-related potentials and their neural origins MS Gazzaniga (Eds) An introduction to the event-related potential technique MIT Press Cambridge, Massachusetts 27–33
F Malouin CL Richards PL Jackson F Dumas J Doyon (2003) ArticleTitleBrain activations during motor imagery of locomotor-related tasks: a PET study Hum Brain Mapp 19 47–62 10.1002/hbm.10103 Occurrence Handle10.1002/hbm.10103 Occurrence Handle12731103
JC Masdeu (2001) ArticleTitleNeuroimaging and gait Adv Neurol 87 83–89 Occurrence Handle1:STN:280:DC%2BD3Mris12hsw%3D%3D Occurrence Handle11347246
H Matsui F Udaka T Miyoshi N Hara A Tamaura M Oda T Kubori K Nishinaka M Kameyama (2005) ArticleTitleThree-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson’s disease Mov Disord 20 1272–1277 10.1002/mds.20520 Occurrence Handle10.1002/mds.20520 Occurrence Handle16007622
T Mima N Sadato S Yazawa T Hanakawa H Fukuyama Y Yonekura H Shibasaki (1999) ArticleTitleBrain structures related to active and passive finger movements in man Brain 122 IssueIDPt 10 1989–1997 10.1093/brain/122.10.1989 Occurrence Handle10.1093/brain/122.10.1989 Occurrence Handle10506099
I Miyai HC Tanabe I Sase H Eda I Oda I Konishi Y Tsunazawa T Suzuki T Yanagida K Kubota (2001) ArticleTitleCortical mapping of gait in humans: a near-infrared spectroscopic topography study Neuroimage 14 1186–1192 10.1006/nimg.2001.0905 Occurrence Handle10.1006/nimg.2001.0905 Occurrence Handle1:STN:280:DC%2BD3Mnks1aqtA%3D%3D Occurrence Handle11697950
H Nakahara K Doya O Hikosaka (2001) ArticleTitleParallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences — a computational approach J Cogn Neurosci 13 626–647 10.1162/089892901750363208 Occurrence Handle10.1162/089892901750363208 Occurrence Handle1:STN:280:DC%2BD3MvmtVGnsg%3D%3D Occurrence Handle11506661
Y Ouchi T Kanno H Okada E Yoshikawa M Futatsubashi S Nobezawa T Torizuka K Tanaka (2001) ArticleTitleChanges in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson’s disease Brain 124 784–792 10.1093/brain/124.4.784 Occurrence Handle10.1093/brain/124.4.784 Occurrence Handle1:STN:280:DC%2BD3M3gtVahug%3D%3D Occurrence Handle11287377
NT Petersen JE Butler V Marchand-Pauvert R Fisher A Ledebt HS Pyndt NL Hansen JB Nielsen (2001) ArticleTitleSuppression of EMG activity by transcranial magnetic stimulation in human subjects during walking J Physiol 537 651–656 10.1111/j.1469-7793.2001.00651.x Occurrence Handle10.1111/j.1469-7793.2001.00651.x Occurrence Handle1:CAS:528:DC%2BD3MXptlKgt70%3D Occurrence Handle11731595
M Plotnik N Giladi Y Balash C Peretz JM Hausdorff (2005) ArticleTitleIs freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann Neurol 57 656–663 10.1002/ana.20452 Occurrence Handle10.1002/ana.20452 Occurrence Handle15852404
CA Porro MP Francescato V Cettolo ME Diamond P Baraldi C Zuiani M Bazzocchi PE di Prampero (1996) ArticleTitlePrimary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study J Neurosci 16 7688–7698 Occurrence Handle1:CAS:528:DyaK28XntFOisrw%3D Occurrence Handle8922425
S Rossignol R Dubuc JP Gossard (2006) ArticleTitleDynamic sensorimotor interactions in locomotion Physiol Rev 86 89–154 10.1152/physrev.00028.2005 Occurrence Handle10.1152/physrev.00028.2005 Occurrence Handle16371596
M Roth J Decety M Raybaudi R Massarelli C Delon-Martin C Segebarth S Morand A Gemignani M Decorps M Jeannerod (1996) ArticleTitlePossible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study Neuroreport 7 1280–1284 Occurrence Handle1:STN:280:DyaK28vhsVGhug%3D%3D Occurrence Handle8817549 Occurrence Handle10.1097/00001756-199605170-00012
K Sacco F Cauda L Cerliani D Mate S Duca GC Geminiani (2006) ArticleTitleMotor imagery of walking following training in locomotor attention. The effect of “the tango lesson” Neuroimage 32 1441–1449 10.1016/j.neuroimage.2006.05.018 Occurrence Handle10.1016/j.neuroimage.2006.05.018 Occurrence Handle1:STN:280:DC%2BD28rht1OgsA%3D%3D Occurrence Handle16861008
C Sahyoun A Floyer-Lea H Johansen-Berg PM Matthews (2004) ArticleTitleTowards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements Neuroimage 21 568–575 10.1016/j.neuroimage.2003.09.065 Occurrence Handle10.1016/j.neuroimage.2003.09.065 Occurrence Handle1:STN:280:DC%2BD2c%2FptFKqtQ%3D%3D Occurrence Handle14980558
M Schubert A Curt L Jensen V Dietz (1997) ArticleTitleCorticospinal input in human gait: modulation of magnetically evoked motor responses Exp Brain Res 115 234–246 10.1007/PL00005693 Occurrence Handle10.1007/PL00005693 Occurrence Handle1:STN:280:DyaK2szmvVSrtw%3D%3D Occurrence Handle9224852
KM Stephan GR Fink RE Passingham D Silbersweig AO Ceballos-Baumann CD Frith RS Frackowiak (1995) ArticleTitleFunctional anatomy of the mental representation of upper extremity movements in healthy subjects J Neurophysiol 73 373–386 Occurrence Handle1:STN:280:DyaK2M3jtVensQ%3D%3D Occurrence Handle7714579
M Suzuki I Miyai T Ono I Oda I Konishi T Kochiyama K Kubota (2004) ArticleTitlePrefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study Neuroimage 23 1020–1026 10.1016/j.neuroimage.2004.07.002 Occurrence Handle10.1016/j.neuroimage.2004.07.002 Occurrence Handle15528102
M Vidailhet F Stocchi JC Rothwell PD Thompson BL Day DJ Brooks CD Marsden (1993) ArticleTitleThe Bereitschaftspotential preceding simple foot movement and initiation of gait in Parkinson’s disease Neurology 43 1784–1788 Occurrence Handle1:STN:280:DyaK2c%2FitVejtA%3D%3D Occurrence Handle8414032
S Yazawa H Shibasaki A Ikeda K Terada T Nagamine M Honda (1997) ArticleTitleCortical mechanism underlying externally cued gait initiation studied by contingent negative variation Electroencephalogr Clin Neurophysiol 105 390–399 10.1016/S0924-980X(97)00034-9 Occurrence Handle10.1016/S0924-980X(97)00034-9 Occurrence Handle1:STN:280:DyaK1c%2FivFaqsA%3D%3D Occurrence Handle9363005
Acknowledgement
This research was supported by the Stichting Internationaal Parkinson Fonds (to MB & BB). BB was also supported by an NWO VIDI research grant (#91776352). IT was supported by the Dutch Science Foundation (NWO: VIDI grant no. 452-03-339).
Author information
Authors and Affiliations
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bakker, M., Verstappen, C., Bloem, B. et al. Recent advances in functional neuroimaging of gait. J Neural Transm 114, 1323–1331 (2007). https://doi.org/10.1007/s00702-007-0783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-007-0783-8