Abstract
Background
The appropriate management of delayed cerebral ischemia (DCI) after aneurysmal subarachnoid hemorrhage (aSAH) remains uncertain. We aimed to evaluate the effect of implementing a standardized protocol for detection and management of DCI after aSAH on cerebral infarction and functional outcome.
Methods
We studied two cohorts of aSAH patients, one before (pre-implementation cohort: January 2012 to August 2014) and one after (post-implementation cohort: January 2016 to July 2018) implementation of a multidisciplinary approach, with standardized neurological and radiological assessment and risk-based medical treatment of DCI. We assessed the presence of new hypodensities on CT within 6 weeks after aSAH and categorized cerebral infarction into overall and DCI-related infarctions (hypodensities not within 48 h after IA repair and not attributable to aneurysm occlusion or intraparenchymal hematoma). Functional outcome was assessed at 3 months using the extended Glasgow outcome scale (eGOS), dichotomized into unfavorable (eGOS: 1–5) and favorable (eGOS: 6–8). We calculated odds ratios (OR) with corresponding 95% confidence intervals (CI’s), and adjusted for age, WFNS grade, Fisher score, and treatment modality (aOR).
Results
In the post-implementation (n = 158) versus the pre-implementation (n = 143) cohort the rates for overall cerebral infarction were 29.1% vs 46.9% (aOR: 0.41 [0.24–0.69]), for DCI-related cerebral infarction 17.7% vs. 31.5% (aOR: 0.41 [0.23–0.76]), and for unfavorable functional outcome at 3 months 37.3% vs. 53.8% (aOR: 0.30 [0.17–0.54]). For patients with DCI, the rates for unfavorable functional outcomes at 3 months in the post-implementation versus the pre-implementation cohort were 42.3% vs. 77.8% (aOR: 0.1 [0.03–0.27]).
Conclusions
A multidisciplinary approach with more frequent and standardized neurological assessment, standardized CT and CT perfusion monitoring, as well as tailored application of induced hypertension and invasive rescue therapy strategies, is associated with a significant reduction of cerebral infarction and unfavorable functional outcome after aneurysmal aSAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DCI is an important determinant for neurological outcomes after aSAH. Although it was traditionally assumed that DCI is predominantly caused by angiographic vasospasm, more recent studies highlighted that DCI is a multifactorial phenomenon, related to several pro-ischemic pathomechanisms [13]. This concept is supported by the fact that not all patients with vasospasm develop DCI, and not all patients who develop DCI have vasospasm.
To focus on cerebral hemodynamics instead of only angiographic vasospasm, CT perfusion imaging can be applied for detection and even prediction of DCI and may be especially useful in aSAH patients who are neurologically not assessable, but previous studies reported conflicting results in this respect [18]. For treatment of patients with clinical or radiological features of DCI, hypertension often is induced to ameliorate neurological worsening and prevent cerebral infarction and poor neurological outcomes [9, 16]. In patients with DCI refractory to induce hypertension, additional rescue therapy strategies, such as intra-arterial administration of nimodipine, are often considered [5, 7]. However, such treatments carry a risk of major complications and their risk–benefit ratio remains uncertain. We aimed to determine the effect of implementing a multidisciplinary approach, with frequent and standardized clinical assessment, standardized monitoring with CT perfusion, and a medical instead of an invasive initial step in the treatment of DCI on radiologically proven infarction and functional outcome in patients with aSAH.
Methods
Patient population
We studied one cohort before (pre-implementation cohort) and one after (post-implementation cohort) implementing a standardized management protocol (see below). We included all aSAH patients who underwent either surgical or endovascular intracranial aneurysm repair within 24 h after admission.
Definitions
Clinical DCI was defined as a decrease in the level of consciousness (decrease in 2 points in the GCS) or an increase of 2 points in the National Institute of Health Stroke Scale or development of a new focal deficit lasting for at least 1 h and not explained by other factors (i.e., systemic complications and hydrocephalus) [17].
Radiological DCI was defined as a 1.5-fold prolongation of mean transit time (MTT) values, compared to baseline in serial CT perfusion imaging [3]. Severe angiographic vasospasm was defined as narrowing of the arterial diameter of > 70% from the baseline on digital subtraction angiography.
DCI-related cerebral infarctions were considered in the presence of new hypodensities within 6 weeks after the primary aSAH ictus but not within 48 h after IA repair and not attributable to surgical or endovascular treatment nor to intraparenchymal hematoma [17].
We regarded thromboembolic infarctions occurring during the intra-arterial catheter treatment with nimodipine and up to 48 h after catheter removal as nimodipine-catheter associated infarctions. Accordingly, any new intracranial hemorrhage during the intra-arterial nimodipine-catheter treatment with nimodipine was related to the necessary anticoagulation and thus, considered catheter associated.
Functional outcome was assessed at 3 months using dichotomized extended Glasgow outcome scale (eGOS 1–5: unfavorable and eGOS 6–8: favorable).
DCI detection and management in the pre-implementation and post-implementation cohorts
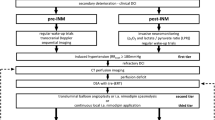
Between January 2012 and August 2014 (pre-implementation cohort), there was no multidisciplinary consented protocol for the management of aSAH patients, who were admitted to our department. Management decisions were usually taken by the treating neurosurgeon or neuroradiologist on an individual basis, and patients were predominately treated according to the presence of angiographic vasospasm. In retrospect, the management in the pre-implementation cohort may be summarized as follows: Patients were (A) neurologically assessed in the intensive or intermediate care unit during daily rounds by neurosurgical residents and staff and (B) underwent digital subtraction angiography on days 6 to 9 after aneurysm repair or immediately upon clinical deterioration for the assessment of the presence of angiographic vasospasm (Figs. 1 and 2a). Clinical deterioration was defined as a new neurological deficit (aphasia and/or paresis) or a decline in consciousness. Angiographic vasospasm was regarded as clinically relevant in case of clinical deterioration suggestive of DCI or evidence of new hypodensities on unenhanced CT scans. Induced hypertension, if at all, was not consistently applied and was not part of a tailored and escalating treatment approach. Patients with severe, progressive, or clinically relevant vasospasm received an intra-arterial catheter for continuous nimodipine administration as first-line treatment. Angiographic vasospasm was monitored by a follow-up digital subtraction angiography after 72 h.
Clinical and radiological DCI monitoring and screening protocol. Abbreviations: CCT, cranial computed tomography; CTA, CT-angiography; DSA, digital subtraction angiography; i.a., intra-arterial; MTT, mean transit time; Neuro exam, neurological examination every 2 h; PCT, CT perfusion; SAH, subarachnoid hemorrhage
Algorithms for the management of patients with DCI. A DCI management algorithm in the pre-implementation cohort. B Escalating treatment protocol for patients with DCI in the post-implementation cohort. Abbreviations: CCT, cranial computed tomography; DSA, digital subtraction angiography; i.a., intra-arterial; MTT, mean transit time; PCT, perfusion computed tomography
In fall 2015, the authors (NE, CG, ENP, JK) developed and implemented an interdisciplinary protocol for standardized detection and management of DCI (see below) in line with their previously reported studies [6, 10]. Following a transition period, from January 2016 onward, all aSAH patients admitted to our department (post-implementation cohort) were routinely discussed in a multidisciplinary team of neurosurgeons, neuroradiologists, and intensive care specialists, and management decisions in case of deterioration were taken based on the protocol and multidisciplinary discussion. The standardized detection protocol included thorough neurological examinations every 2–4 h by dedicated neurocritical care residents in combination with a novel CT perfusion screening protocol (Fig. 1). CT perfusion measurements were performed 6–12 h after aneurysm treatment, on days 3 or 4 as well as days 9 to 11 after aSAH, or in case of clinical features of DCI [6, 10, 17]. For patients who were and remained in perfect condition, the last CT perfusion was omitted. Additionally, digital subtraction angiographies were performed on admission and on days 6 to 9 after aSAH ictus and in case of clinical deterioration from DCI or CT perfusion deficits. In the case of persistent clinical signs of DCI, severe CT perfusion impairment or severe angiographic vasospasm, the patients in the post-implementation cohort were treated by means of a standardized, escalating treatment protocol: First, induced hypertension with a targeted systolic blood pressure of > 180 mmHg; second, solitary intra-arterial nimodipine bolus applications during catheter angiography; and third angiographic application of an intra-arterial catheter for continuous nimodipine administration over 48 h with CT perfusion imaging in between each escalating step (Fig. 2b).
The NEWTON-II study was a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group, efficacy and safety study comparing single-dose intraventricular nimodipine microparticles to oral nimodipine with respect to functional outcome and the incidence of DCI and cerebral infarction in patients after aSAH [2]. During this second phase, our center participated between August 2017 and March 2018 in this study, and patients admitted to our department during this time period were screened and if eligible enrolled and treated within the NEWTON-II study.
Statistical analysis
Statistical analyses were performed using R version 3.6.2 [The R Foundation]. Continuous data were presented as mean (SD). Categorical data are presented as numbers (%). P-values for continuous data were derived from t-tests, and P-values for categorical data are derived from chi-square tests. Significance was defined at a level of P < 0.05. A logistic regression model was used to analyze the effects of the multidisciplinary and tailored escalating approach on functional and radiological outcomes. After calculation of crude odds ratios for functional and radiological outcomes, the model was adjusted for age, WFNS grade, Fisher score, and treatment modality.
Results
We included 143 patients in the pre-implementation cohort and 158 in the post-implementation cohort (Table 1). In both the pre- and the post-implementation period, 9 patients died before IA repair and were therefore not included. Of the 158 patients in the post-implementation cohort, 8 participated in the NEWTON-II study, with equal distribution into the interventional and control arm [2]. In the pre-implementation cohort, 69 (48.3%) patients had angiographic vasospasm, of which 45 (31.5%) had clinically relevant vasospasms. Of those patients, 3 (4.3%) received induced hypertension, 2 (2.9%) single-shot, and 38 (55.1%) continuous intra-arterial nimodipine treatment (Table 1). In the post-implementation cohort, 126 (79.7%) patients developed any radiological DCI, of which 124 (98.4%) were treated by means of induced hypertension, 59 (46.8%) with single, and 14 (11.1%) with continuous intra-arterial nimodipine treatment. None of the patients in the pre- and post-implementation cohort developed renal complications. Especially, in view of the repeated CT perfusion imaging, we observed no renal failure associated with the regular use of contrast agents in the post-implementation cohort.
Radiological and functional outcomes
Any cerebral infarction occurred in 67 (46.9%) patients in the pre-implementation cohort and in 46 (29.1%) in the post-implementation cohort (aOR: 0.41 [0.24–0.69]). DCI-related cerebral infarction occurred in 45 (31.5%) patients in the pre-implementation cohort and in 28 (17.7%) patients in the post-implementation cohort, aOR was 0.41 [0.23–0.76] (Table 2, Figs. 3 and 5).
Nimodipine-catheter-associated infarctions in the pre-implementation cohort versus the post-implementation cohort occurred in 4 (10.5%) patients vs. 1 (7.1%) patient.
In the pre-implementation cohort, 77 (53.8%) patients had an unfavorable functional outcome versus 57 (37.3%) in the post-implementation cohort (aOR: 0.30 [0.17–0.54]) (Table 2, Figs. 4a and 5).
Stacked histograms of functional outcome. A Overall functional outcome at 3 months. B Functional outcome in patients with DCI at 3 months. Distribution of scores according to the extended Glasgow Outcome Scale between the pre-implementation cohort (January 2012–August 2014) and the post-implementation cohort (January 2016– July 2018) for patients with DCI. Abbreviations: eGOS, extended Glasgow Outcome Scale. *P < 0.05; **P < 0.01
In patients who developed DCI, in the pre-implementation cohort, unfavorable outcome was present in 35 (77.8%) patients, compared to 52 (42.3%) patients in the post-implementation cohort (aOR: 0.1 [0.03–0.27]) (Table 2, Figs. 4b and 5).
The exclusion of the 8 patients who were enrolled in the NEWTON-II trial from the analysis did not show any effect on the results (Table 3).
Effect of DCI treatment in patients with exclusive radiological DCI
Of all patients in the post-implementation cohort who received DCI treatment, 24 patients were treated based on radiological DCI only and exclusively received induced hypertension. Compared to patients in the pre-implementation cohort with no evidence of clinical DCI or hemodynamically relevant macrovascular spasms, the rates of overall and DCI-related cerebral infarction at 6 weeks after SAH ictus were lower in the post-implementation cohort, which was more distinct after adjustment for age, WFNS grade, Fisher score, and treatment modality (Fig. 6). These patients tended to have lower rates of unfavorable functional outcomes at discharge as well as at 3 months. This effect was more pronounced after adjustment for age, WFNS grade, Fisher score, and treatment modality (Fig. 6).
Discussion
Our study revealed that a multidisciplinary approach, with frequent and standardized clinical assessment, standardized monitoring with CT perfusion, and staged and escalating management instead of invasive primary step in treatment in case of DCI was associated with a significantly improved radiological and functional outcome after aSAH, compared to aSAH patients who were, in the absence of a dedicated multidisciplinary standard operating procedure.
Previous studies reporting conflicting results on the role of DCI detection and/or rescue therapy after SAH: A study on multimodal and invasive monitoring for DCI and a prespecified algorithm for endovascular rescue treatment (transluminal balloon angioplasty and/or continuous intraarterial nimodipine) reported good functional outcomes at 3 months in 35.5% in those patients who received endovascular rescue treatment [19]. Our protocol did not only yield a lower rate of patients requiring intra-arterial catheter treatment (11.1% vs. 20.1%) but also a higher rate of favorable functional outcomes in the subgroup of patients in the post-implementation cohort who received continuous intra-arterial nimodipine treatment (57.1% vs. 35.5%) [19].
Another study on frequent and early endovascular treatment of angiographic vasospasm reported a reduced incidence of DCI-related cerebral infarction and an improved functional outcome [12]. However, the reported rates of DCI-related infarction (20.8%) and unfavorable outcomes (defined as mRS > 2, 44%) in this study were higher than those in our post-implementation cohort (17.7% for cerebral infarction and 34% for the unfavorable outcome, considering a comparable unfavorable outcome cutoff at eGOS < 5).
The improved outcome in the post-implementation cohort may be explained by the strict multidisciplinary approach, the regular clinical assessment by dedicated personnel, the frequent CTP measurements, or the more stepwise approach in patients with DCI compared to the pre-implementation cohort, or a combination of these factors. More vigilant multidisciplinary management may in itself result in better outcomes, independent of specific interventions used in the protocol [15]. Additionally, the implementation of a standardized protocol may lead to a higher dedication of the treating team in terms of a more thorough clinical and neurological monitoring. Alternatively, the radiological surveillance implemented in our protocol for detection of DCI using serial CT perfusion imaging, especially in patients who are clinically not assessable, may have also resulted in earlier detection of DCI and thereby earlier rescue therapy for DCI. This is supported by previous CT perfusion studies in the setting of aSAH, since CT perfusion may detect both macro- and microcirculatory disturbances prior to clinical manifestation of DCI [4, 18]. Furthermore, a risk-based, escalating rescue therapy in patients with clinical and/or radiological DCI may have resulted in a beneficial risk–benefit ratio and thereby improved outcomes, compared to sole angiographic vasodilation as a first-line therapy [7]. Although the benefit of induced hypertension for the management of DCI after SAH is unproven, [8] it carries a risk of complications. Moreover, intra-arterial treatment strategies harbor an additional risk for new cerebral infarcts due to thromboembolism and arterial dissection [1]. The higher Fisher score may partly explain the higher rate of clinical DCI in the post-implementation cohort. Nevertheless, in view of the better outcome in the post-implementation cohort in our study compared to standardized management protocols of previous studies, it seems more likely that not only the protocol implementation itself but especially the diagnostic and therapeutic means in our protocol may have had a meaningful effect on radiological and functional outcome [12, 19].
We acknowledge several limitations: First, our study comprises a retrospective analysis of prospectively collected data and one could argue that our findings are no more than a “self-fulfilling prophecy.” Second, as our protocol essentially comprises multiple interacting “interventions,” we cannot further analyze the individual contribution of each of these components to the outcomes. Third, patients in the post-implementation cohort may have higher risks to develop DCI and poor neurological outcomes. This is partly reflected in the higher rate of clinical DCI in the post-implementation cohort. We addressed this by adjusting our logistic regression models for these factors, which resulted in an even more pronounced effect toward improved radiological and functional outcomes in the post-implementation cohort. Fourth, it is often debated whether the radiation exposure of serial CT perfusion imaging and repeated patient transports are justified to improve outcomes in aSAH patients. In line with previous studies and in view of the absolute risk reduction for cerebral infarction and unfavorable outcome in our data, our study indicates a reasonable risk–benefit ratio [11, 14]. Additionally, the inclusion of patients treated within the NEWTON-II trial may have seemed to introduce some bias in the post-implementation cohort with respect to functional outcomes. However, our exploratory analyses without these 8 patients did not reveal any effect on our results, which is most likely explained by the equal distribution of patients into the two treatment arms and especially because the NEWTON-II trial protocol complied with our own institutional standard. Since these patients were treated in accordance with our standardized protocol described here, we refrained from omitting these patients from the complete dataset. Lastly, we cannot entirely rule out the effect of improved outcome after aSAH due to advances in aSAH management in general over time. However, since the two observational periods differed only by 3 years, we do not foresee time trends in aSAH outcome as a major contributor to our findings.
Conclusion
Our data highlight that protocol-based, rigorous detection and management strategies for DCI may reduce the rate of unnecessary invasive rescue procedures and, consequently, complication rates. To avoid excessive use of CTP, it is important to tailor the indication for CTP imaging to the risk of DCI and/or neurological status of SAH patients. A cluster randomized trial may provide more definitive data on the associations we found.
References
Adami D, Berkefeld J, Platz J, Konczalla J, Pfeilschifter W, Weidauer S, Wagner M (2019) Complication rate of intraarterial treatment of severe cerebral vasospasm after subarachnoid hemorrhage with nimodipine and percutaneous transluminal balloon angioplasty: worth the risk? J Neuroradiol 46:15–24. https://doi.org/10.1016/j.neurad.2018.04.001
Carlson AP, Hanggi D, Wong GK, Etminan N, Mayer SA, Aldrich F, Diringer MN, Schmutzhard E, Faleck HJ, Ng D, Saville BR, Bleck T, Grubb R Jr, Miller M, Suarez JI, Proskin HM, Macdonald RL, Investigators N (2020) Single-dose intraventricular nimodipine microparticles versus oral nimodipine for aneurysmal subarachnoid hemorrhage. Stroke 51:1142–1149. https://doi.org/10.1161/STROKEAHA.119.027396
Cremers CH, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJ, Velthuis BK, Vergouwen MD (2014) CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 34:200–207. https://doi.org/10.1038/jcbfm.2013.208
Cremers CH, Vos PC, van der Schaaf IC, Velthuis BK, Vergouwen MD, Rinkel GJ, Dankbaar JW (2015) CT perfusion during delayed cerebral ischemia after subarachnoid hemorrhage: distinction between reversible ischemia and ischemia progressing to infarction. Neuroradiology 57:897–902. https://doi.org/10.1007/s00234-015-1543-3
de Oliveira Manoel AL, Goffi A, Marotta TR, Schweizer TA, Abrahamson S, Macdonald RL (2016) The critical care management of poor-grade subarachnoid haemorrhage. Crit Care 20:21. https://doi.org/10.1186/s13054-016-1193-9
Etminan N, Beseoglu K, Heiroth HJ, Turowski B, Steiger HJ, Hanggi D (2013) Early perfusion computerized tomography imaging as a radiographic surrogate for delayed cerebral ischemia and functional outcome after subarachnoid hemorrhage. Stroke 44:1260–1266. https://doi.org/10.1161/STROKEAHA.111.675975
Francoeur CL, Mayer SA (2016) Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 20:277. https://doi.org/10.1186/s13054-016-1447-6
Gathier CS, van den Bergh WM, van der Jagt M, Verweij BH, Dankbaar JW, Muller MC, Oldenbeuving AW, Rinkel GJE, Slooter AJC, Group HS (2018) Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a randomized clinical trial. Stroke 49:76–83. https://doi.org/10.1161/STROKEAHA.117.017956
Haegens NM, Gathier CS, Horn J, Coert BA, Verbaan D, van den Bergh WM (2018) Induced hypertension in preventing cerebral infarction in delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 49:2630–2636. https://doi.org/10.1161/STROKEAHA.118.022310
Hanggi D, Turowski B, Beseoglu K, Yong M, Steiger HJ (2008) Intra-arterial nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid hemorrhage: influence on clinical course and cerebral perfusion. AJNR Am J Neuroradiol 29:1053–1060. https://doi.org/10.3174/ajnr.A1005
Ivanidze J, Charalel RA, Shuryak I, Brenner D, Pandya A, Kallas ON, Kesavabhotla K, Segal AZ, Simon MS, Sanelli PC (2017) Effects of radiation exposure on the cost-effectiveness of CT angiography and perfusion imaging in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 38:462–468. https://doi.org/10.3174/ajnr.A5034
Jabbarli R, Pierscianek D, Rolz R, Darkwah Oppong M, Kaier K, Shah M, Taschner C, Monninghoff C, Urbach H, Beck J, Sure U, Forsting M (2019) Endovascular treatment of cerebral vasospasm after subarachnoid hemorrhage: more is more. Neurology 93:e458–e466. https://doi.org/10.1212/WNL.0000000000007862
Macdonald RL (2014) Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 10:44–58. https://doi.org/10.1038/nrneurol.2013.246
Sanelli PC, Pandya A, Segal AZ, Gupta A, Hurtado-Rua S, Ivanidze J, Kesavabhotla K, Mir D, Mushlin AI, Hunink MG (2014) Cost-effectiveness of CT angiography and perfusion imaging for delayed cerebral ischemia and vasospasm in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 35:1714–1720. https://doi.org/10.3174/ajnr.A3947
Siner JM, Connors GR (2015) Protocol-based care versus individualized management of patients in the intensive care unit. Semin Respir Crit Care Med 36:870–877. https://doi.org/10.1055/s-0035-1566157
Vergouwen MD, Etminan N, Ilodigwe D, Macdonald RL (2011) Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 31:1545–1553. https://doi.org/10.1038/jcbfm.2011.56
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275
Washington CW, Zipfel GJ, Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H (2011) Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care 15:312–317. https://doi.org/10.1007/s12028-011-9594-8
Weiss M, Conzen C, Mueller M, Wiesmann M, Clusmann H, Albanna W, Schubert GA (2019) Endovascular rescue treatment for delayed cerebral ischemia after subarachnoid hemorrhage is safe and effective. Front Neurol 10:136. https://doi.org/10.3389/fneur.2019.00136
Acknowledgements
The authors sincerely acknowledge the unrestrained support of all members of the medical and nursing staff of the intensive care unit and the Department of Neuroradiology of the University Hospital Mannheim for the implementation of our standardized aSAH management protocol.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study adheres to ethical guidelines and was approved by the local ethics committee of the Medical Faculty Mannheim of the Ruprecht-Karls-University, Heidelberg, Germany (study ID: 2019-822R).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical intensive care
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdulazim, A., Küppers, C., Hackenberg, K.A.M. et al. Multidisciplinary and standardized management of patients with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir 164, 2917–2926 (2022). https://doi.org/10.1007/s00701-022-05347-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05347-y