Abstract
Objective
To compare outcomes between patients with primary external ventricular device (EVD)–driven treatment of intracranial hypertension and those with primary intraparenchymal monitor (IP)–driven treatment.
Methods
The CENTER-TBI study is a prospective, multicenter, longitudinal observational cohort study that enrolled patients of all TBI severities from 62 participating centers (mainly level I trauma centers) across Europe between 2015 and 2017. Functional outcome was assessed at 6 months and a year. We used multivariable adjusted instrumental variable (IV) analysis with “center” as instrument and logistic regression with covariate adjustment to determine the effect estimate of EVD on 6-month functional outcome.
Results
A total of 878 patients of all TBI severities with an indication for intracranial pressure (ICP) monitoring were included in the present study, of whom 739 (84%) patients had an IP monitor and 139 (16%) an EVD. Patients included were predominantly male (74% in the IP monitor and 76% in the EVD group), with a median age of 46 years in the IP group and 48 in the EVD group. Six-month GOS-E was similar between IP and EVD patients (adjusted odds ratio (aOR) and 95% confidence interval [CI] OR 0.74 and 95% CI [0.36–1.52], adjusted IV analysis). The length of intensive care unit stay was greater in the EVD group than in the IP group (adjusted rate ratio [95% CI] 1.70 [1.34–2.12], IV analysis). One hundred eighty-seven of the 739 patients in the IP group (25%) required an EVD due to refractory ICPs.
Conclusion
We found no major differences in outcomes of patients with TBI when comparing EVD-guided and IP monitor–guided ICP management. In our cohort, a quarter of patients that initially received an IP monitor required an EVD later for ICP control. The prevalence of complications was higher in the EVD group.
Protocol
The core study is registered with ClinicalTrials.gov, number NCT02210221, and the Resource Identification Portal (RRID: SCR_015582).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In severe traumatic brain injury (TBI), intracranial pressure (ICP) is frequently monitored to guide treatment of intracranial hypertension [5].
Two main groups of devices are used to monitor ICP [21]. Intraparenchymal (IP) monitors are usually inserted in the intensive care unit (ICU) or the operating room (OR) by drilling a hole in the skull, piercing the meninges and inserting the thin catheter in the brain parenchyma of the right frontal region (or tailored to the expected maximum swelling area). External ventricular devices (EVDs) are usually inserted in the OR by drilling a larger burr hole above Kocher’s point and inserting the catheter in the lateral ventricles.
There is considerable practice variation with respect to the choice of monitoring device [5]. From a pathophysiological perspective, the use of an EVD instead of an IP monitor would offer more ICP control and therefore result in a better outcome [21]. This is because using an EVD enables not only intracranial pressure monitoring, but also ICP-lowering therapy: drainage of cerebrospinal fluid (CSF) that may obviate the need for other ICP lowering treatments, including decompressive craniectomy (DC). One of the major drawbacks of EVD use is a higher risk of complications, notably drain-related infections, compared to parenchymal monitors [21]. Furthermore, EVDs may lead to slit ventricles, and increase the risk of surgical complications such as hematoma formation.
A meta-analysis of our group which pooled the results of all available studies until 2018 [3, 21] showed no benefit in terms of mortality or functional outcome when using EVD instead of IP monitors. This meta-analysis overturned the result of the only randomized controlled trial (RCT) on the topic, which showed superiority of EVDs in terms of mortality and functional outcome [11]. A more recent retrospective analysis showed worse outcomes in patients treated with a primary EVD [3]. Explanations of these contradictory findings could be confounding by indication present in the retrospective observational studies, albeit adjusted with state-of-the-art statistical methods [1, 3, 10], and the limited generalizability of the RCT [11]. Historically, treatment guidelines for TBI indicated EVDs as “third tier” therapies [4]. In the most recent edition of the guidelines, their place in the severe treatment strategy is no longer stated. The only current recommendation is that CSF should be drained continuously.
Studying an isolated intervention is difficult in TBI patients, because of the strong interdependence of individual treatment modalities aimed at lowering intracranial hypertension, as well as the heterogeneity of the patient population [23]. This sometimes leads to confusing guideline recommendations because of the difficulty in generating robust evidence in TBI [22]. However, this variation does provide opportunities for comparative effectiveness research (CER) [12, 17, 24]. CER exploits practice variation by taking advantage of the “natural experiment” that occurs when patients go to different hospitals, each with their own treatment preferences. Analyzing the effect of treatment preference instead of actual treatment a patient received minimizes confounding by indication. Therefore, the treatment effect estimate from this analysis should have a lower risk of bias [7, 13].
Within a large prospective observational study, CENTER-TBI, we aimed to compare outcomes between patients with an EVD and patients with an IP monitor as the primary ICP monitoring modality. We hypothesized that patients receiving an EVD would have a better outcome due to the option to drain CSF, decreasing the need for third tier therapies and decompressive craniectomies.
Methods
Patient population
The CENTER-TBI study is a prospective, multicenter, longitudinal observational cohort study that enrolled patients of all TBI severities. TBI patients presenting within 24 h after injury with a clinical indication for a brain CT scan to one of the 62 participating study sites in Europe (mainly level 1 trauma centers), or referred from another hospital to the participating study site, were eligible for this study. Extensive details and the study design are available in a previous publication [14, 19]. For this study we included patients of all TBI severities admitted to the intensive care unit (ICU) with an indication for ICP monitoring.
Patient characteristics
Baseline characteristics extracted from the CENTER-TBI database were age, sex, total injury severity score, pupillary reactivity, the most reliable Glasgow Coma Scale (GCS) and motor GCS score, pupillary reactivity, and injury cause. Furthermore, from the first CT scan, the following features were extracted: the presence of a skull fracture, epidural or subdural hematoma or traumatic subarachnoid hemorrhage, intraventricular hemorrhage, compression of basal cisterns, and midline shift.
Outcomes and aims
The primary aims of this study were to assess 6-month mortality and unfavorable functional outcome in the EVD group compared to the IP monitor group. The co-primary outcomes were, therefore, mortality at 6 months and unfavorable functional outcome, as defined by the 6-month Glasgow Outcome Scale-Extended (GOS-E) of 4 or less (Supplementary Table 1).
The secondary outcomes were as follows: (a) the median daily therapy intensity level (TIL) for the first 12 days in the ICU together with the median ICP per day; the TIL is a validated measure of how much therapy a patient requires in order to control ICP; (b) hospital and ICU length of stay (LOS); (c) the use of secondary DC for refractory intracranial hypertension; (d) the use of other third tier therapies (barbiturate coma, hypothermia); (e) the risk of overall complications, defined as infection (meningitis/ventriculitis) and delayed hematoma (new intracerebral hematoma on follow-up radiological studies) or monitoring device malfunction; (f) mortality in the ICU and in the hospital; (g) the prevalence of cross-over (i.e., IP monitor patients eventually receiving an EVD); (h) ratio of time points with ICP above 20 mmHg and above 25 mmHg out of all recorded ICP time points. ICP was registered hourly during ICU stay for the first 10 days, and on days 12, 14, and 21. We only included “secondary” DCs, excluding DCs performed before insertion of the monitoring device, for a primary space-occupying lesion or for signs of intracranial hypertension on the first CT scan.
Sensitivity analyses
The main analysis mirrored an “intention-to-treat” analysis from a trial. We defined the groups based on the first monitor received. Therefore, the entire subgroup of patients that received an EVD later on due to refractory hypertension was considered part of the IP monitor group even though they harbored both monitors.
As a sensitivity analysis, we analyzed all outcomes “as-treated,” by defining groups as “IP monitor only” and “EVD at any timepoint.”
As a second sensitivity analysis, we re-ran all analyses with 12-month mortality and unfavorable outcome as co-primary outcomes.
A final sensitivity analysis was the “complete case” analysis, which included only patients without missing data.
Data analysis
Baseline characteristics were compared between patients who received an EVD or IP monitor with Student’s t-tests, Mann–Whitney U tests, chi-squared tests, or Fisher’s exact test. Furthermore, to provide a summary measure of “baseline risk” for each group, the probabilities of 6-month mortality and unfavorable outcomes were predicted based on baseline characteristics and the base IMPACT model [16] using logistic regression. The median predicted probability per treatment group was calculated, with the interquartile range (IQR).
Because we assume missing at random (MAR), we performed multiple imputation on the data to obtain 5 datasets. Outcomes were included in the imputation model (less than 15% missing).
To analyze which patient characteristics affected the choice for an EVD or IP monitor, logistic regression analysis was performed, with IP monitor as the reference outcome category. All relevant predictors from the descriptive analysis were included, but only those with a p-value below 0.2 in the full model were retained in the final model. To take center effects into account, the model was extended with a nested random intercept for center. For both models, the Nagelkerke R2 was calculated, to assess the proportion of variance in treatments that was explained by the different models.
The effects of EVD versus IP on outcome were estimated based on two methods.
First, an instrumental variable approach was used, with center preference for EVD over IP as instrument, both unadjusted and adjusted for confounders.
Second, we performed multivariable adjustment using variables that differed between the groups at baseline. To assess the effect of EVD versus IP on unfavorable outcome (GOS-E < 5), mortality, and decompressive craniectomy, logistic regression analysis was used. For the effect on length of hospital or ICU stay, a quasi-Poisson regression analysis was performed.
The adjusted IV analysis was considered the main analysis and these effect size estimates were reported. This choice was motivated by the considerable effect of adding a random intercept for center to the analysis of factors associated with choice of monitor. Using adjusted IV analysis minimized confounding by indication present in non-randomized data. Regarding outcomes for which the number of events was too small to use IV analysis (use of third tier therapies, complications, ratio of time points with ICP above 20 mmHg and above 25 mmHg out of all recorded ICP time points), we consider the main effect size estimate the one provided by multivariable confounder adjustment.
Instrumental variable (IV) analysis [7, 13] can theoretically correct for observed and unobserved confounders. To be valid, three assumptions should be met [13]. The relevance assumption was confirmed by logistic regression analysis. Most centers included in the study were level I trauma centers actively involved in TBI research with considerable experience in monitoring modalities, we therefore considered the exclusion assumption met. Furthermore, we verified this assumption in a previously published paper by our group [9]. For the exchangeability assumption, we considered previous research showing that in TBI, correlation between known confounders and center is low [6]. Given these arguments, we judged our instrument (center) for the IV analysis as being of moderate strength [15]. Clinical centers are an accepted and valid instrument in the scientific literature [6]. Centers including less than 10 patients were excluded. Our group’s previous research shows that a cut-off of 10 patients is a valid choice [7].
Results
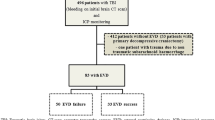
A total of 2138 patients were admitted to the ICU in the CENTER-TBI cohort, with median age of 49 years; 36% of whom were mild TBI (GCS 13–15). A total of 878 ICU patients were included in the present study. Of these, 739 (84%) patients received an IP monitor and 139 (16%) patients received an EVD (Table 1). For the main analysis (“intention-to-treat”), we included in the “IP monitor” group all patients who received an IP monitor first, including 187 who received an EVD for CSF drainage at later stages in their ICP management course. The instrumental variable analysis, which excluded centers with less than 10 patients, included 639 patients with an IP monitor and 115 with an EVD (Supplementary Table 2, Supplementary Fig. 1).
Baseline characteristics
Patients included were predominantly male (74% in the IP monitor and 76% in the EVD group), with a median age of 46 years in the IP and 48 years in the EVD group (Table 1). Most common trauma mechanisms were road traffic accidents in 48% of cases and falls in 38% of all cases. The median GCS on presentation was 6 in the IP group and 5.5 in the EVD group. The majority of patients had equal and reactive pupils (75% in the IP monitor group and 64% in the EVD group). There were no statistically significant differences in baseline characteristics except for the higher prevalence of unreactive pupils in the EVD group in the subset of patients included in the IV analysis (Supplementary Table 2). In both the complete sample and in the sample for the IV analysis, the predicted mortality was higher in the EVD group (Table 1, Supplementary Table 2). The vast majority of devices were inserted within the first 12 h, with more than one-third being inserted in the first 6 h (Table 2, Supplementary Fig. 3).
Choice of device
The significant predictors for use of EVD over IP in the fixed-effects model were one unreactive pupil (OR [95% CI] 1.96 [1.05–3.65]), emergency intracranial surgery (OR [95% CI] 2.44 [1.45–4.09]), and emergency intracranial and extracranial surgery (OR [95% CI] 4.08 [1.71–9.71], and also significant in the random-effects analysis: OR [95% CI] 3.38 [1.03–11.08]) (Supplementary Table 3). The Nagelkerke R2 of the model with patient characteristics alone was 0.28. The addition of a random intercept for center conditional increased the Nagelkerke R2 to 0.57 (Supplementary Table 3). Of the EVD patients, 24 received a ventricular device with a mounted pressure sensor; these were excluded from the intention-to-treat analysis and added to the as-treated analysis in the EVD group.
Therapy and length of stay
The median daily ICP was significantly different between the two groups, but the difference was clinically irrelevant, with 11 mmHg in the IP monitor group and 12 mmHg in the EVD group (p = 0.01) (Table 2, Fig. 1A). Duration of ICP monitoring was 6.3 days in the IP monitor group (IQR 3.4–10.6) and 7.5 days (IQR 4.3–12.3) in the EVD group. The ratio of high ICP measurements, defined as the number of instances with an ICP measured above 20 or 25 divided by the total number of time points measured, was not different between the two groups (Table 2).
The median daily therapy intensity level (TIL) was not different between the two groups, even when excluding CSF drainage (median [IQR] 5 [3,4,5,6,7,8,9] in the IP group and 7 [3.5–11] in the EVD group) (Table 2, Fig. 1B). A median of 75 ml CSF was drained daily in the EVD group (Table 2).
The mean hospital length of stay (HLOS) and ICU length of stay (ICU LOS) were higher in the EVD group (Table 3), with the mean number of days spent in the ICU being 70% higher in the EVD group, rate ratio = 1.7, 95% CI [1.34–2.12], adjusted IV analysis.
A total of 187 patients (25%) who were primarily monitored with an IP monitor crossed over and required an EVD later on during ICP treatment due to refractory high ICP.
Outcomes
The 6-month GOSE (dichotomized) did not differ between the two groups, EVD versus IP OR 0.74 and 95% CI [0.36–1.52], adjusted IV analysis (Fig. 2, Table 3). Mortality at 6 months did not differ significantly between the two groups (aOR and 95% CI 1.03 [0.40–2.48]).
The need for decompressive craniectomy was similar between groups, but the analysis was underpowered (aOR and 95% CI 0.68 [95% CI: 0.11–2.45], adjusted IV analysis) (Table 3).
The need for any third-tier therapies was not different between the two groups (aOR 1.35 and 95% CI [0.90–2.04], multivariable regression) (Table 3).
The rate of overall complications was similar between groups (aOR 1.58 and 95% CI [0.74–3.41], multivariable regression). The risk of a delayed hematoma was significantly higher in the EVD group (aOR 2.04 and 95% CI [1.28–3.24]) (Table 3).
Because of the higher risk of delayed hematoma in both the main and sensitivity analyses, we decided to further explore the relationship between the amount of CSF drained and the risk of delayed hematoma using multivariable logistic regression. The analysis showed no association between either daily median drained volume or total drained volume and the risk of developing a delayed hematoma (aOR [95% CI] 1.0 [0.9–1.1] for both covariates). The only variable associated with the risk of developing a delayed hematoma was having bilateral fixed pupils at baseline (aOR [95% CI] 4.2 [1.35–13.69]).
We also explored the relationship between CSF drainage and the need for third tier therapies, adjusting for covariates associated with the need for higher TIL, reported in a previous work [8]. When comparing the IP monitoring group with the “early EVD” (primary intention) group and “late EVD” group (patients who received an EVD after an IP monitor), the late EVD group had a significantly higher need for third tier therapies (aOR [95% CI] 2.26 [1.43–3.4]).
Sensitivity analyses
The “as-treated” analysis involved moving the 187 patients that received an EVD at a later time point after IP insertion due to refractory high ICPs to the “EVD” group. This left 552 patients in the IP monitor group and 336 in the EVD group. The 336 patients in the EVD group included 10 patients who had both monitors from the beginning and who were excluded from the “intention-to-treat” analysis. At baseline, the EVD group had more subdural hematomas, the basal cisterns were more often compressed, and both the predicted mortality and predicted unfavorable outcome at 6 months were higher.
The EVD group had higher overall TILs, a higher ratio of time points with an ICP > 20 or 25 mmHg and needed overall more third tier therapies. Interestingly, while this change did affect the coefficients, and the EVD group had an overall higher rate of complications (aOR [95% CI] 2.17 [1.61–2.94], multivariable analysis) and a higher need for third tier therapies (aOR [95% CI] 2.22 [1.66–2.97], multivariable analysis), both 6-month mortality and unfavorable functional outcome were similar between groups (mortality aOR [95% CI] 1.55 [0.67–3.49] and unfavorable functional outcome 1.04 [0.52–2.13], adjusted IV analysis) (Supplementary Table 4).
The sensitivity analysis which employed the 12-month GOS-E supported our conclusions, with all results remaining the same, no difference in mortality or unfavorable functional outcome for both the “intention-to-treat” as for the “as-treated” analyses. The level of missing covariate data for clinical baseline variables was between 0 and 6.4% (Supplementary Table 5). For the baseline CT covariates, the level varied between 13.9 and 15.5%. The complete case sensitivity analysis also revealed similar effect size estimates, with no difference in terms of primary outcome (Supplementary Table 6).
Discussion
Our study found no major differences in functional outcome or mortality when comparing ICP monitoring with EVDs versus IP monitors to guide ICP management. Draining cerebrospinal fluid concomitantly with ICP measurement does not confer benefit in itself for ultimate clinical outcome. The risk of a delayed hematoma was higher and length of stay was prolonged in the patients managed with EVDs, but this did not translate to worse functional outcomes. A quarter of patients initially treated with IP monitoring had to cross over to EVD because of refractory high ICP during their ICP management period.
We performed a CER approach using data from one of the largest prospective TBI cohorts to date to compare the outcomes of the EVD group with those of the IP monitor group. The evidence so far is poor and contradictory [3, 11, 21], making practice variation the rule, not the exception [20]. While this would not be an issue if indeed the two methods are equally effective [21], reports suggesting the superiority of one or the other treatment modalities [3, 11] made further research necessary. In our data, mortality and functional outcomes did not differ between the two groups.
Our meta-analysis of all studies up to 2018 shows no difference in terms of mortality or functional outcome between patients given IP monitors and those given EVDs [21]. The only RCT on this topic [11], including 122 patients, with relatively low risk of bias, showed lower mortality, better functional outcome, and a reduced need for decompressive craniectomy in patients receiving an EVD. A more recent study, using state-of-the-art statistical methods for confounder adjustment [3], showed significantly worse functional and neuropsychological outcomes in patients with EVDs. Of interest, we re-ran the meta-analysis using the new data provided by Bales et al. together with data from the current study. We found no difference, either in terms of mortality or functional outcome. From a methodological standpoint, however, internal validity of included studies should always take precedence when interpreting the results of any meta-analysis, and from this point of view, despite the pooled result, the study by Liu et al. remains the most methodologically sound [11]. The only issue with this RCT is its limited generalizability given the likely unrepresentative sample of 122 patients (relatively young patients, less than 20% being above 60 years of age and 25% of the sample had a GCS above 8).
We had hypothesized a lower therapy intensity level [25] (TIL) in the EVD group given that drainage of CSF has an impact on lowering ICP. Less need for ICP-lowering therapy when ICP is monitored with an EVD has been suggested by previous research [11]. The TIL was not significantly different between the two groups, but the median ICP showed a statistically significant difference. However, this difference was of 1 mmHg, rendering it clinically irrelevant. Furthermore, ICP control was achieved to the same extent in both groups. We hypothesized that the presence of an EVD, actively draining CSF, might prevent the use of additional aggressive ICP-lowering treatments. This, however, was also not sustained by our data showing no differences in the use of third tier therapies between the two groups. One-fourth of patients in the IP monitoring group did eventually cross over and required an EVD for ICP control. The risk of complications, such as infection, has often been quoted as a reason to not use EVDs to monitor and treat TBI patients [21]. In the main analysis, we did not find any difference in terms of infections between the IP and EVD group. In the sensitivity analysis, however, the risk of infection was higher in the EVD group, as expected, but this did not translate into worse clinical outcomes.
For the “average” TBI patient presenting with an indication for monitoring, choosing an EVD instead of an intraparenchymal monitor does not appear to lead to better functional outcome or lower mortality. There is a higher risk of delayed hematoma, but we found no evidence to suggest this complication was directly related to the magnitude of the intervention itself, the amount of drained CSF. The unanswered question still remains if patients that actually develop refractory high ICP will benefit from early drainage. This is a slightly different question and patient selection than our present study. It also requires a better characterization of the phenotype of patients that develop refractory ICP. Our cohort included patients who lent themselves to ICP control with relative ease. In deciding whether to monitor with an IP monitor or an EVD, however, clinicians should take into account the fact that in our cohort, one-fourth of patients crossed over, and required an EVD later on during ICP monitoring for refractory high ICP. The “as treated” EVD group had overall higher TILs and need for third tier therapies. This might be related to confounding by indication present in the data, and is nothing more than an association. We cannot exclude the potential explanation that placing an EVD later on during ICP treatment leads to a higher need for third tier therapies. We were unfortunately unable to model the relationship “time to late EVD,” but this finding suggests clinicians should have a low threshold for placing EVDs early on during ICP treatment.
Both EVDs and IP monitors inform treatment and ICP-lowering therapies based on these monitoring modalities lead to similar outcomes. Our data shows that, when EVDs are used as a first intention, there is no difference in outcome when compared to IP monitors. When starting with an IP monitor, however, a quarter of patients do require this step (EVDs) in their ICP management protocol. We feel this very high cross-over rate should raise awareness to the potential use of EVDs, even though on a group level they do not improve the primary outcomes. Further, if both approaches (IP and EVD) are equally effective in terms of outcome, costs become an issue. We note that EVDs are considerably cheaper than IP devices.
Limitations
Despite CENTER-TBI including a generous cohort of TBI patients, we were only able to include 136 patients with EVDs in the main analysis. In the start-up phase of our study, we conducted “provider profiling” of participating centers. Within these self-administered questionnaires, we profiled the “standard of care” across participating centers. Whereas 60% of centers indicated using both modalities and 8% indicated using only EVDs for monitoring [5], we could not confirm this in the core data of the patients included. We used IV analysis for our primary outcomes using center as a moderate strength instrument, but despite explaining a considerable amount of variation, there likely remains a significant amount of residual confounding. For most patients in our study, most of the measured ICP values were under 15, indicating that both groups of patients mostly had controlled ICP. ICP was also not measured using the “high resolution” package [2], which might further confound results, as “instantaneous” measurements were not available and the influence of short bursts of high ICP could not be evaluated.
An issue not dealt with in our study is whether CSF should be drained continuously or intermittently. The current guidelines recommend continuous drainage, but this recommendation is based on two observational studies which include a small number of patients, leaving this question still open for debate [4, 18].
We recognize the limitations of our study and of its interpretation. If anything, the study illustrates the challenges and complexity of a CER design within an observational study in the specific field of TBI, and as such may serve to stimulate debate and reflection on the use of more advanced methodologies for future research.
Conclusion
We found no major differences in clinical outcomes of patients undergoing IP monitor- or EVD-based ICP treatment, using a comparative effectiveness design. A quarter of patients who received an IP monitor as a first intention eventually required an EVD. The prevalence of complications was higher in the EVD group.
References
Aiolfi A, Khor D, Cho J, Benjamin E, Inaba K, Demetriades D (2018) Intracranial pressure monitoring in severe blunt head trauma: does the type of monitoring device matter? J Neurosurg 128:828–833
Åkerlund CA, Donnelly J, Zeiler FA, Helbok R, Holst A, Cabeleira M, Güiza F, Meyfroidt G, Czosnyka M, Smielewski P, Stocchetti N, Ercole A, Nelson DW (2020) Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: a CENTER-TBI high-resolution group study. PLoS ONE 15:e0243427. https://doi.org/10.1371/journal.pone.0243427
Bales JW, Bonow RH, Buckley RT, Barber J, Temkin N, Chesnut RM (2019) Primary external ventricular drainage catheter versus intraparenchymal ICP monitoring: outcome analysis. Neurocrit Care 31:11–21. https://doi.org/10.1007/s12028-019-00712-9
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J (2017) Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery 80:6–15. https://doi.org/10.1227/neu.0000000000001432
Cnossen MC, Huijben JA, van der Jagt M, Volovici V, van Essen T, Polinder S, Nelson D, Ercole A, Stocchetti N, Citerio G, Peul WC, Maas AIR, Menon D, Steyerberg EW, Lingsma HF, investigators C-T, (2017) Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: a survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit Care 21:233
Cnossen MC, Lingsma HF, Maas AI, Menon DK, Steyerberg EW (2015) Estimating treatment effectiveness of intracranial pressure monitoring in traumatic brain injury. Crit Care Med 43:e599
Cnossen MC, van Essen TA, Ceyisakar IE, Polinder S, Andriessen TM, van der Naalt J, Haitsma I, Horn J, Franschman G, Vos PE, Peul WC, Menon DK, Maas AI, Steyerberg EW, Lingsma HF (2018) Adjusting for confounding by indication in observational studies: a case study in traumatic brain injury. Clin Epidemiol 10:841–852. https://doi.org/10.2147/clep.S154500
Huijben JA, Dixit A, Stocchetti N, Maas AIR, Lingsma HF, van der Jagt M, Nelson D, Citerio G, Wilson L, Menon DK, Ercole A (2021) Use and impact of high intensity treatments in patients with traumatic brain injury across Europe: a CENTER-TBI analysis. Crit Care 25:78. https://doi.org/10.1186/s13054-020-03370-y
Huijben JA, Wiegers EJA, Lingsma HF, Citerio G, Maas AIR, Menon DK, Ercole A, Nelson D, van der Jagt M, Steyerberg EW, Helbok R, Lecky F, Peul W, Birg T, Zoerle T, Carbonara M, Stocchetti N (2020) Changing care pathways and between-center practice variations in intensive care for traumatic brain injury across Europe: a CENTER-TBI analysis. Intensive Care Med 46:995–1004. https://doi.org/10.1007/s00134-020-05965-z
Kasotakis G, Michailidou M, Bramos A, Chang Y, Velmahos G, Alam H, King D, De Moya MA (2012) Intraparenchymal vs extracranial ventricular drain intracranial pressure monitors in traumatic brain injury: less is more? J Am Coll Surg 214:950–957. https://doi.org/10.1016/j.jamcollsurg.2012.03.004
Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J, Ren G (2015) External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg 83:794–800
Lohr KN (2010) Comparative effectiveness research methods: symposium overview and summary. Med Care 48:S3-6
Lousdal ML (2018) An introduction to instrumental variable assumptions, validation and estimation. Emerg Themes Epidemiol 15:1. https://doi.org/10.1186/s12982-018-0069-7
Maas AI, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, Hill S, Legrand V, Sorgner A, Participants C-T, Investigators (2015) Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76:67–80
Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH (2006) Instrumental variables: application and limitations. Epidemiology 17:260–267. https://doi.org/10.1097/01.ede.0000215160.88317.cb
Roozenbeek B, Lingsma HF, Lecky FE, Lu J, Weir J, Butcher I, McHugh GS, Murray GD, Perel P, Maas AI, Steyerberg EW, International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury Study G, Corticosteroid Randomisation After Significant Head Injury Trial C, Trauma A, Research N (2012) Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit Care Med 40:1609–1617
Schneeweiss S, Seeger JD, Jackson JW, Smith SR (2013) Methods for comparative effectiveness research/patient-centered outcomes research: from efficacy to effectiveness. J Clin Epidemiol 66:S1-4
Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, Bayir H, Jackson EK, Kochanek PM (2004) Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma 21:1113–1122
Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, Ercole A, Kunzmann K, Lanyon L, Lecky F, Lingsma H, Manley G, Nelson D, Peul W, Stocchetti N, von Steinbüchel N, Vande Vyvere T, Verheyden J, Wilson L, Maas AIR, Menon DK (2019) Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. The Lancet Neurology 18:923–934. https://doi.org/10.1016/s1474-4422(19)30232-7
Volovici V, Ercole A, Citerio G, Stocchetti N, Haitsma IK, Huijben JA, Dirven CMF, van der Jagt M, Steyerberg EW, Nelson D, Cnossen MC, Maas AIR, Polinder S, Menon DK, Lingsma HF (2019) Variation in guideline implementation and adherence regarding severe traumatic brain injury treatment: a CENTER-TBI survey study in Europe. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.01.116
Volovici V, Huijben JA, Ercole A, Stocchetti N, Dirven CMF, van der Jagt M, Steyerberg EW, Lingsma HF, Menon DK, Maas AIR, Haitsma IK (2019) Ventricular drainage catheters versus intracranial parenchymal catheters for intracranial pressure monitoring-based management of traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma 36(7):988–995. https://doi.org/10.1089/neu.2018.6086
Volovici V, Steyerberg EW (2021) Lost in translation between evidence and recommendations: expert opinion is needed to define “level I”. World Neurosurg. https://doi.org/10.1016/j.wneu.2021.03.095
Volovici V, Steyerberg EW, Cnossen MC, Haitsma IK, Dirven CMF, Maas AIR, Lingsma HF (2019) Evolution of evidence and guideline recommendations for the medical management of severe traumatic brain injury. J Neurotrauma 36:3183–3189. https://doi.org/10.1089/neu.2019.6474
Williams CM, Skinner EH, James AM, Cook JL, McPhail SM, Haines TP (2016) Comparative effectiveness research for the clinician researcher: a framework for making a methodological design choice. Trials 17:406
Zuercher P, Groen JL, Aries MJ, Steyerberg EW, Maas AI, Ercole A, Menon DK (2016) Reliability and validity of the therapy intensity level scale: analysis of clinimetric properties of a novel approach to assess management of intracranial pressure in traumatic brain injury. J Neurotrauma 33:1768–1774. https://doi.org/10.1089/neu.2015.4266
Acknowledgements
The CENTER-TBI ICU WP6 participants and ICU ONLY investigators:
Cecilia Åkerlund1, Krisztina Amrein2, Nada Andelic3, Lasse Andreassen4, Gérard Audibert5, Philippe Azouvi6, Maria Luisa Azzolini7, Ronald Bartels8, Ronny Beer9, Bo-Michael Bellander10, Habib Benali11, Maurizio Berardino12, Luigi Beretta7, Erta Beqiri13, Morten Blaabjerg14, Stine Borgen Lund15, Camilla Brorsson16, Andras Buki17, Manuel Cabeleira18, Alessio Caccioppola19, Emiliana Calappi19, Maria Rosa Calvi7, Peter Cameron20, Guillermo Carbayo Lozano21, Ana M. Castaño-León22, Simona Cavallo12, Giorgio Chevallard13, Arturo Chieregato13, Mark Coburn24, Jonathan Coles25, Jamie D. Cooper26, Marta Correia27, Endre Czeiter17, Marek Czosnyka18, Claire Dahyot-Fizelier28, Paul Dark29, Véronique De Keyser30, Vincent Degos11, Francesco Della Corte31, Hugo den Boogert8, Bart Depreitere32, Dula Dilvesi33, Abhishek Dixit34, Jens Dreier35, Guy-Loup Dulière36, Erzsébet Ezer37, Martin Fabricius38, Kelly Foks39, Shirin Frisvold40, Alex Furmanov41, Damien Galanaud11, Dashiell Gantner20, Alexandre Ghuysen42, Lelde Giga43, Jagos Golubovic33, Pedro A. Gomez22, Francesca Grossi31, Deepak Gupta44, Iain Haitsma45, Eirik Helseth46, Peter J. Hutchinson47, Stefan Jankowski48, Faye Johnson49, Mladen Karan33, Angelos G. Kolias47, Daniel Kondziella38, Evgenios Koraropoulos34, Lars-Owe Koskinen50, Noémi Kovács51, Ana Kowark24, Alfonso Lagares22, Steven Laureys52, Didier Ledoux52, Aurelie Lejeune53, Roger Lightfoot54, Alex Manara55, Costanza Martino56, Hugues Maréchal36, Julia Mattern57, Catherine McMahon58, Tomas Menovsky30, Benoit Misset52, Visakh Muraleedharan59, Lynnette Murray20, Ancuta Negru60, Virginia Newcombe34, József Nyirádi2, Fabrizio Ortolano19, Jean-François Payen61, Vincent Perlbarg11, Paolo Persona62, Anna Piippo-Karjalainen63, Horia Ples60, Inigo Pomposo21, Jussi P. Posti64, Louis Puybasset65, Andreea Radoi66, Arminas Ragauskas67, Rahul Raj63, Jonathan Rhodes68, Sophie Richter34, Saulius Rocka67, Cecilie Roe69, Olav Roise70,71, Jeffrey V. Rosenfeld72, Christina Rosenlund73, Guy Rosenthal41, Rolf Rossaint24, Sandra Rossi62, Juan Sahuquillo66, Oddrun Sandrød75, Oliver Sakowitz57,75, Renan Sanchez-Porras75, Kari Schirmer-Mikalsen74,76, Rico Frederik Schou77, Peter Smielewski18, Abayomi Sorinola78, Emmanuel Stamatakis34, Nina Sundström79, Riikka Takala80, Viktória Tamás78, Tomas Tamosuitis81, Olli Tenovuo64, Matt Thomas55, Dick Tibboel73, Christos Tolias83, Tony Trapani19, Cristina Maria Tudora60, Peter Vajkoczy75, Shirley Vallance20, Egils Valeinis 43, Zoltán Vámos37, Gregory Van der Steen30, Roel P. J. van Wijk66, Alessia Vargiolu23, Emmanuel Vega52, Anne Vik76,85, Rimantas Vilcinis81, Victor Volovici45, Petar Vulekovic33, Guy Williams34, Stefan Winzeck34, Stefan Wolf86, Alexander Younsi57, Frederick A. Zeiler34,87, Agate Ziverte43, Hans Clusmann88, Daphne Voormolen89, Jeroen T.J.M. van Dijck90, Thomas A. van Essen90
1Department of Physiology and Pharmacology, Section of Perioperative Medicine and Intensive Care, Karolinska Institutet, Stockholm, Sweden
2János Szentágothai Research Centre, University of Pécs, Pécs, Hungary
3Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway
4Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway
5Department of Anesthesiology & Intensive Care, University Hospital Nancy, Nancy, France
6Raymond Poincare hospital, Assistance Publique – Hopitaux de Paris, Paris, France
7Department of Anesthesiology & Intensive Care, S Raffaele University Hospital, Milan, Italy
8Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands
9Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria
10Department of Neurosurgery & Anesthesia & intensive care medicine, Karolinska University Hospital, Stockholm, Sweden
11Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France
12Department of Anesthesia & ICU, AOU Città della Salute e della Scienza di Torino—Orthopedic and Trauma Center, Torino, Italy
13NeuroIntensive Care, Niguarda Hospital, Milan, Italy
14Department of Neurology, Odense University Hospital, Odense, Denmark
15Department of Public Health and Nursing, Faculty of Medicine and health Sciences, Norwegian University of Science and Technology, NTNU, Trondheim, Norway
16Department of Surgery and Perioperative Science, Umeå University, Umeå, Sweden
17Department of Neurosurgery, Medical School, University of Pécs, Hungary and Neurotrauma Research Group, János Szentágothai Research Centre, University of Pécs, Hungary
18Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
19Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
20ANZIC Research Centre, Monash University, Department of Epidemiology and Preventive Medicine, Melbourne, Victoria, Australia
21Department of Neurosurgery, Hospital of Cruces, Bilbao, Spain
22Department of Neurosurgery, Hospital Universitario 12 de Octubre, Madrid, Spain
23NeuroIntensive Care, ASST di Monza, Monza, Italy
24Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany
25Department of Anesthesia & Neurointensive Care, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK
26School of Public Health & PM, Monash University and The Alfred Hospital, Melbourne, Victoria, Australia
27Radiology/MRI department, MRC Cognition and Brain Sciences Unit, Cambridge, UK
28Intensive Care Unit, CHU Poitiers, Potiers, France
29University of Manchester NIHR Biomedical Research Centre, Critical Care Directorate, Salford Royal Hospital NHS Foundation Trust, Salford, UK.
30Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium
31Department of Anesthesia & Intensive Care, Maggiore Della Carità Hospital, Novara, Italy
32Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium
33Department of Neurosurgery, Clinical centre of Vojvodina, Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia
34Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
35Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
36Intensive Care Unit, CHR Citadelle, Liège, Belgium
37Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary
38Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark
39Department of Neurology, Erasmus MC, Rotterdam, the Netherlands
40Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway
41Department of Neurosurgery, Hadassah-hebrew University Medical center, Jerusalem, Israel
42Emergency Department, CHU, Liège, Belgium
43Neurosurgery clinic, Pauls Stradins Clinical University Hospital, Riga, Latvia
44Department of Neurosurgery, Neurosciences Centre & JPN Apex trauma centre, All India Institute of Medical Sciences, New Delhi-110029, India
45Department of Neurosurgery, Erasmus MC, Rotterdam, the Netherlands
46Department of Neurosurgery, Oslo University Hospital, Oslo, Norway
47Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital & University of Cambridge, Cambridge, UK
48Neurointensive Care , Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
49Salford Royal Hospital NHS Foundation Trust Acute Research Delivery Team, Salford, UK
50Department of Clinical Neuroscience, Neurosurgery, Umeå University, Umeå, Sweden
51Hungarian Brain Research Program—Grant No. KTIA_13_NAP-A-II/8, University of Pécs, Pécs, Hungary
52Cyclotron Research Center , University of Liège, Liège, Belgium
53Department of Anesthesiology-Intensive Care, Lille University Hospital, Lille, France
54Department of Anesthesiology & Intensive Care, University Hospitals Southhampton NHS Trust, Southhampton, UK
55Intensive Care Unit, Southmead Hospital, Bristol, Bristol, UK
56Department of Anesthesia & Intensive Care,M. Bufalini Hospital, Cesena, Italy
57Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany
58Department of Neurosurgery, The Walton centre NHS Foundation Trust, Liverpool, UK
59Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden
60Department of Neurosurgery, Emergency County Hospital Timisoara , Timisoara, Romania
61Department of Anesthesiology & Intensive Care, University Hospital of Grenoble, Grenoble, France
62Department of Anesthesia & Intensive Care, Azienda Ospedaliera Università di Padova, Padova, Italy
63Department of Neurosurgery, Helsinki University Central Hospital
64Division of Clinical Neurosciences, Department of Neurosurgery and Turku Brain Injury Centre, Turku University Hospital and University of Turku, Turku, Finland
65Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France
66Neurotraumatology and Neurosurgery Research Unit (UNINN), Vall d'Hebron Research Institute, Barcelona, Spain
67Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania
68Department of Anaesthesia, Critical Care & Pain Medicine NHS Lothian & University of Edinburg, Edinburgh, UK
69Department of Physical Medicine and Rehabilitation, Oslo University Hospital/University of Oslo, Oslo, Norway
70Division of Orthopedics, Oslo University Hospital, Oslo, Norway
71Institute of Clinical Medicine, Faculty of Medicine, University of Olso, Oslo, Norway
72National Trauma Research Institute, The Alfred Hospital, Monash University, Melbourne, Victoria, Australia
73Department of Neurosurgery, Odense University Hospital, Odense, Denmark
74Department of Anaesthesiology and Intensive Care Medicine, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
75Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany
76Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU, Trondheim, Norway
77Department of Neuroanesthesia and Neurointensive Care, Odense University Hospital, Odense, Denmark
78Department of Neurosurgery, University of Pécs, Pécs, Hungary
79Department of Radiation Sciences, Biomedical Engineering, Umeå University, Umeå, Sweden
80Perioperative Services, Intensive Care Medicine and Pain Management, Turku University Hospital and University of Turku, Turku, Finland
81Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania
82Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children’s Hospital, Rotterdam, The Netherlands
83Department of Neurosurgery, Kings college London, London, UK
84Neurologie, Neurochirurgie und Psychiatrie, Charité – Universitätsmedizin Berlin, Berlin, Germany
85Department of Neurosurgery, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
86Department of Neurosurgery, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
87Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
88Department of Neurosurgery, University Hospital of Aachen, Aachen, Germany
89Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands
90Dept. of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands and Dept. of Neurosurgery, Medical Center Haaglanden, The Hague, The Netherlands
Funding
The European Commission, Seventh Framework Program, grant number 602150, provided support in the form of funding for the CENTER-TBI project, not only for this study in particular. The Hannelore Kohl Stiftung (Germany), OneMind (USA), and Integra LifeSciences Corporation (USA) provided support for the CENTER-TBI project in the form of funding, not only for this study in particular. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee of the Erasmus MC Rotterdam and of all 62 participant centers in the CENTER-TBI project and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Brain trauma.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Volovici, V., Pisică, D., Gravesteijn, B.Y. et al. Comparative effectiveness of intracranial hypertension management guided by ventricular versus intraparenchymal pressure monitoring: a CENTER-TBI study. Acta Neurochir 164, 1693–1705 (2022). https://doi.org/10.1007/s00701-022-05257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05257-z