Abstract
Background
Subarachnoid hemorrhage (SAH) can lead to acute hydrocephalus (AH). AH pathophysiology is classically attributed to an obstruction of the arachnoid granulations by blood. Recent findings in rodents suggest that after intraventricular hemorrhage, AH is related to cerebrospinal fluid (CSF) hypersecretion by the choroid plexus (CP), as it can be reduced by intracerebroventricular (ICV) injection of bumetanide.
Objective
Here, we investigated if and how CSF hypersecretion and/or CSF outflow disorders contribute to post-SAH hydrocephalus.
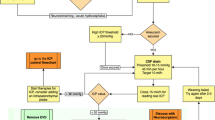
Methods
Ninety-four Wistar rats were used. SAH was induced by the endovascular perforation technique. The presence of AH was confirmed by magnetic resonance imaging (MRI), and rats with AH were randomly assigned to 4 groups: control group, superior sagittal sinus (SSS) thrombosis to block CSF reabsorption, ICV injection of saline, and ICV injection of bumetanide to decrease CSF secretion. Clinical outcome was evaluated with a neuroscore. A second MRI was performed 24 h later to evaluate the ventricular volume.
Results
Fifty percent of rats that survived SAH induction had AH. Their ventricular volume correlated well to the functional outcome after 24 h (r = 0.803). In rats with AH, 24 h later, ventricular volume remained equally increased in the absence of any further procedure. Similarly, ICV injection of saline or SSS thrombosis had no impact on the ventricular volume. However, ICV injection of bumetanide reduced AH by 35.9% (p = 0.002).
Conclusion
In rodents, post-SAH hydrocephalus is may be due to hypersecretion of CSF by the CP, as it is limited by ICV injection of bumetanide. However, we cannot exclude other mechanisms involved in post-SAH acute hydrocephalus.



Similar content being viewed by others
Abbreviations
- AH:
-
Acute hydrocephalus
- CP:
-
Choroid plexus
- DCI:
-
Delayed cerebral ischemia
- CSF:
-
Cerebrospinal fluid
- EVD:
-
External ventricular drain
- ICV:
-
Intracerebroventricular
- IVH:
-
Intraventricular hemorrhage
- PHH:
-
Post-hemorrhagic hydrocephalus
- SAH:
-
Subarachnoid hemorrhage
- SSS:
-
Superior sagittal sinus
References
Akins PT, Guppy KH (2021) Does impaired glymphatic drainage cause glymphedema? A review tailored to neurocritical care and neurosurgery. Neurocrit Care. https://doi.org/10.1007/s12028-021-01224-1
Black PM (1986) Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurosurgery 18(1):12–16
Chen S, Luo J, Reis C, Manaenko A, Zhang J (2017) Hydrocephalus after subarachnoid hemorrhage: pathophysiology, diagnosis, and treatment. Biomed Res Int 2017:8584753
Connolly ES, Rabinstein AA, Carhuapoma JR et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43(6):1711–1737
Delpire E, Gagnon KB (2019) Elusive role of the Na-K-2Cl cotransporter in the choroid plexus. Am J Physiol Cell Physiol 316(4):C522–C524
Foerch C, Arai K, Jin G, Park K-P, Pallast S, van Leyen K, Lo EH (2008) Experimental model of warfarin-associated intracerebral hemorrhage. Stroke 39(12):3397–3404
Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz J-L, Emery E, Touze E, Vivien D, Gauberti M (2014) Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 45(10):3092–3096
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634 discussion 635
Goulay R, Flament J, Gauberti M et al (2017) Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke 48(8):2301–2305
Gregoriades JMC, Madaris A, Alvarez FJ, Alvarez-Leefmans FJ (2019) Genetic and pharmacological inactivation of apical Na+-K+-2Cl- cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am J Physiol Cell Physiol 316(4):C525–C544
Guo D, Wilkinson DA, Thompson BG, Pandey AS, Keep RF, Xi G, Hua Y (2017) MRI characterization in the acute phase of experimental subarachnoid hemorrhage. Transl Stroke Res 8(3):234–243
Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J (1989) Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke 20(6):747–753
Karimy JK, Zhang J, Kurland DB et al (2017) Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med 23(8):997–1003
Liu E, Peng X, Ma H, Zhang Y, Yang X, Zhang Y, Sun L, Yan J (2021) The involvement of aquaporin-4 in the interstitial fluid drainage impairment following subarachnoid hemorrhage. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2020.611494
Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES (2002) Ventriculostomy-related infections: a critical review of the literature. Neurosurgery 51(1):170–181 discussion 181-182
Okubo S, Strahle J, Keep RF, Hua Y, Xi G (2013) Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke 44(2):547–550
Plog BA, Nedergaard M (2018) The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13:379–394
Röttger C, Bachmann G, Gerriets T, Kaps M, Kuchelmeister K, Schachenmayr W, Walberer M, Wessels T, Stolz E (2005) A new model of reversible sinus sagittalis superior thrombosis in the rat: magnetic resonance imaging changes. Neurosurgery 57(3):573–580 discussion 573–580
Shishido H, Zhang H, Okubo S, Hua Y, Keep RF, Xi G (2016) The effect of gender on acute hydrocephalus after experimental subarachnoid hemorrhage. Acta Neurochir Suppl 121:335–339
Sokołowski W, Barszcz K, Kupczyńska M, Czubaj N, Skibniewski M, Purzyc H (2018) Lymphatic drainage of cerebrospinal fluid in mammals - are arachnoid granulations the main route of cerebrospinal fluid outflow? Biologia (Bratisl) 73(6):563–568
Steffensen AB, Oernbo EK, Stoica A, Gerkau NJ, Barbuskaite D, Tritsaris K, Rose CR, MacAulay N (2018) Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat Commun 9(1):2167
van Asch CJJ, van der Schaaf IC, Rinkel GJE (2010) Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 31(1):67–70
Wan Y, Hua Y, Garton HJL, Novakovic N, Keep RF, Xi G (2019) Activation of epiplexus macrophages in hydrocephalus caused by subarachnoid hemorrhage and thrombin. CNS Neurosci Ther 25(10):1134–1141
Wan S, Wei J, Hua Y, Koduri S, Keep RF, Xi G, Pandey AS (2020) Cerebrospinal fluid from aneurysmal subarachnoid hemorrhage patients leads to hydrocephalus in nude mice. Neurocrit Care. https://doi.org/10.1007/s12028-020-01031-0
Zuurbier SM, van den Berg R, Troost D, Majoie CB, Stam J, Coutinho JM (2015) Hydrocephalus in cerebral venous thrombosis. J Neurol 262(4):931–937
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on CSF Circulation
Rights and permissions
About this article
Cite this article
Metayer, T., Orset, C., Ali, C. et al. Bumetanide lowers acute hydrocephalus in a rat model of subarachnoid hemorrhage. Acta Neurochir 164, 499–505 (2022). https://doi.org/10.1007/s00701-021-05088-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-05088-4