Abstract
Background
Studies on the associations between preoperative cerebral edema, cognitive functioning, and health-related quality of life (HRQOL) in WHO grade I meningioma patients are virtually lacking. We studied the association between preoperative cerebral edema on postoperative cognitive functioning and HRQOL 6 months postoperatively in WHO grade I meningioma patients.
Methods
Twenty-one consecutive WHO grade I meningioma patients, who underwent surgery, were matched individually for age, gender, and educational level to healthy controls. Tumor and edema volume were assessed on preoperative T1- and T2-weighted MRI images, respectively. At least 5 months postoperatively, functional status, cognitive functioning, and HRQOL, using a cognitive test battery and the Short-Form Health Survey (SF-36), were determined. The correlation between preoperative tumor and cerebral edema volume with postoperative cognitive functioning and HRQOL was investigated using Kendall’s tau coefficients.
Results
Compared to healthy controls, patients had lower verbal memory capacity (p = .012), whereas HRQOL was similar to matched healthy controls. In all cognitive domains, postoperative functioning was much lower in patients with preoperative cerebral edema than in those without. There were significant correlations between preoperative cerebral edema and tumor volume and postoperative cognitive functioning. Preoperative cerebral edema and/or tumor volume were not associated with HRQOL.
Conclusions
Our results suggest that WHO grade I meningioma patients with larger volumes of preoperative cerebral edema are more at risk of experiencing limitations in longer-term cognitive functioning than patients with no or less edema preoperatively. This is an important knowledge for neurologists and neurosurgeons treating patients with a meningioma. More studies regarding the effect of peritumoral edema on cognitive functioning in meningioma patients are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with a primary intracranial tumor, cognitive deficits as well as epileptic seizures and their treatment might negatively affect health-related quality of life (HRQOL) [3, 11, 32]. Meningiomas are the most frequently reported intracranial tumors, accounting for approximately one-third of all central nervous system tumors [22]. Although the majority of meningiomas is benign, patients have long-term neurological problems that affect normal daily activities [30].
We have previously shown that many patients with suspected as well as with histologically proven World Health Organization (WHO) grade I meningiomas show subtle cognitive deficits that might be attributed to the tumor itself, the surgical treatment, or the occurrence of seizures and treatment with antiepileptic drugs (AED) [6, 31].
Frequently, meningiomas are an incidental finding in ancillary investigations following head trauma or a routine radiological checkup (e.g., “total body MRI”) [16, 24, 34, 35]. Proper selection for surgery of patients with an incidentally found meningioma is, however, hampered by lack of information concerning preoperative predictors for long-term cognitive functioning and HRQOL. For this group of patients, timing and choice of treatment remains a matter of debate.
Several studies have suggested that tumor-related edema, apart from the tumor itself and tumor-related seizures and their treatment, may have a negative impact on cognitive functioning [15, 28, 29, 34]. The reported incidence of edema in meningioma patients varies from 38 to 90% [13, 21, 23, 25].
The aim of this study is to determine whether there is an association between preoperative cerebral edema and postoperative cognitive functioning and HRQOL at least 5 months following surgery in patients with a WHO grade I meningioma.
Methods and materials
Patients
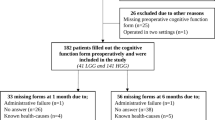
In this cross-sectional study, we included all consecutive patients treated surgically for a meningioma between November 1, 2005 and September 31, 2007, at the VU University Medical Center, which is a tertiary referral center for primary brain tumor patients in the Amsterdam metropolitan area with a total population of approximately 2.4 million people. Excluded were patients with atypical or malignant meningioma (WHO grades II or III) and patients who suffered from one of the following medical conditions, as these may interfere with normal cognitive functioning: other central nervous system (CNS) or non-CNS malignancy, cerebrovascular pathology, congenital CNS malformations, multiple sclerosis, Parkinson’s disease, organic psychosis, dementia, or schizophrenia. Furthermore, patients had to have sufficient proficiency of the Dutch language to be able to carry out the cognitive tests.
The treating physician invited the patients by letter to participate in the study. Of the 28 patients who met the inclusion criteria, 21 agreed to participate in the study; seven patients declined because they considered participation to be too burdensome. After written informed consent was obtained, an appointment was made for cognitive assessment.
Patient characteristics and preoperative symptoms are shown in Table 1.
Healthy controls
For cognitive function, patients were individually matched with healthy controls from the Maastricht Aging Study, which comprises a large cross-sectional study into the biomedical and psychological determinants of cognitive aging of 2000 healthy individuals aged 24 to 81 years [8]. For HRQOL, patient were individually matched with healthy controls from a national study that aimed to translate the Short-Form Health Survey (SF-36) for use among Dutch-speaking residents of The Netherlands [1]. Patients and healthy controls were individually matched with respect to gender, age, and educational level. Educational level was assessed by a Dutch scoring system consisting of an eight-point scale, ranging from unfinished primary education (level 1) to university level (level 8) [5].
The medical ethics committee of the VU University Medical Center approved the study protocol.
Study measures
Functional status
Patients’ capacity to carry out life’s daily activities was assessed by means of the Barthel Activities of Daily Living Index [33]. Scores range from 0 to 20, with higher scores indicating higher levels of functional independence. The Karnofsky Performance Status (KPS) Scale was used as an overall indicator of patients’ level of physical functioning [9]. KPS scores range from 0 to 100, with higher scores indicating higher levels of functioning. Patients’ level of neurologic functioning was assessed by means of Neurological Functional Status Scale, with the score range from 1 to 4; higher scores represent higher levels of neurologic dysfunction [20].
Cognitive functioning
A wide range of cognitive functions was assessed by means of a standardized test battery (see Appendix 1 in the Supplementary Material). Individual cognitive test scores were converted into z-scores using the means and standard deviations (SDs) of the matched healthy controls as a reference. Subsequently, z-scores were transformed into the following six cognitive domains: executive functioning, working memory, verbal memory, attention, information processing speed, and psychomotor speed [11]. Dysfunction in cognitive functioning was defined as a z-score ≥ 1.5 SD below the mean of the healthy controls. The total time required to complete the battery was approximately 60 min. After completing the battery, patients also filled in a visual analogue scale, by which patients reported the amount of effort they had to invest to complete the cognitive testing battery.
Health-related quality of life
Self-reported HRQOL was measured with the Dutch translation of the SF-36 [1]. The SF-36 is composed of 36 items, organized into eight multi-item scales assessing physical functioning (PF), role limitation caused by physical health problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitation caused by emotional problems (RE), and mental health (MH). Raw scores are converted linearly to scales of 0 to 100, with higher scores representing better levels of functioning.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed prior to surgery for each patient. Scans were reviewed by a neuroradiologist (E.S.A.) who was not aware of the patient’s medical history. Data collection included tumor characteristics (tumor volume (cc), volume of edema (cc), total volume of edema and tumor (cc), and tumor localization). Edema was defined as T2-weighted hyperintensity adjacent to the tumor or present in the parenchyma adjacent to the surgical site. If there was no T2-weighted hyperintensity adjacent to the tumor or present in the parenchyma adjacent to the surgical site, we concluded that there was no edema present (example in Figs. 1 and 2). Volumetric measurement of tumor and T2-weighted hyperintensity was done using the BrainLAB iPlan® version 2.6 neuronavigation system (BrainLAB AG, Heimstetten, Germany) to seclude the meningioma.
Statistical analysis
We determined a non-normal distribution because of the small study group. Mann-Whitney U tests were used to test for differences in cognitive functioning and HRQOL between patients and healthy controls. Furthermore, Kendall’s tau coefficients (two-tailed) were used to determine the associations between cognitive functioning or HRQOL and the following factors in both pre- and postoperative MRI scans: edema volume, tumor volume, and combined edema and tumor volume. In the statistical analyses performed using SPSS, version 24 for Windows, statistical significance was set at p < .05 (one-sided, because of the expected deleterious effects of having a meningioma and/or edema).
Results
Sociodemographic and clinical characteristics
As a result of the matching procedure, patients and healthy controls did not differ significantly in age, gender, and educational level (Table 2). Mean tumor volume in meningioma patients with edema was significantly larger than in meningioma patients without edema (37.4 vs 6.0 ml; p = .004).
Cognitive functioning
Patients had a significantly lower verbal memory capacity (p = .012) compared to healthy controls. No statistically significant differences were found between patients and healthy controls in executive functioning, working memory, attention functioning, information processing speed and psychomotor speed. Patients with edema (n = 15) had significantly worse cognitive functioning than patients without edema (n = 6) in all six cognitive domains (Table 3).
Health-related quality of life
Mann-Whitney U test (one-tailed) yielded no significant differences between patients and healthy controls in any of the eight multi-item scales assessing HRQOL (physical functioning p = .180, role limitations caused by physical health problems p = .479, bodily pain p = .237, general health p = .220, vitality p = .192, social functioning p = .448, role limitations caused by emotional problems p = .420 and mental health p = .391). The HRQOL of patients with edema did not differ from patients without edema in any of the eight SF-36 scales (see Table 3).
Associations of tumor and edema volume with cognitive function and HRQOL
Correlational analyses showed significant associations between preoperative peritumoral edema volume and all cognitive domains postoperatively, except verbal memory and attention (Table 4). Considering preoperative tumor volume, analysis showed significant associations with postoperative executive functioning, psychomotor speed, and working memory. When preoperative edema and tumor volume were combined, significant associations were found for all cognitive domains, except for attentional functioning and verbal memory. Furthermore, post hoc analysis yielded significant positive associations between the extent of preoperative edema and preoperative tumor volume (r = .351; p = .030, Kendall’s tau, two-tailed).
No significant associations were established for preoperative edema, preoperative tumor volume, or the combination of edema and tumor volume with HRQOL.
Discussion
We assessed the association between preoperative cerebral edema and postoperative cognitive functioning and HRQOL after surgery for a WHO grade I meningioma and found that patients with preoperative edema had significantly worse cognitive functioning than meningioma patients without preoperative edema after at least 5 months’ follow-up. However, there was no difference in HRQOL between both patient groups 6 months after surgery.
Little is known regarding the effects of edema on cognitive functioning of meningioma patients. Steinvorth et al. evaluated cognitive outcome in patients with skull base meningiomas after fractioned stereotactic radiotherapy and only found a transient decline in memory functioning, 1 day after treatment. The authors concluded that this decline was most likely related to an increase in pre-existing peritumoral edema due to radiotherapy [28]. Tucha et al. showed that preoperative edema was not associated with cognitive functioning 4 to 9 months after surgery [29]. In our study, larger volumes of preoperative edema were significantly associated with lower postoperative cognitive functioning.
Peritumoral edema in meningioma patients is considered to be vasogenic. Possible causative factors for this edema, e.g., tumor volume, tumor location, vascular supply, venous obstruction, histology, vascular endothelial growth factor (VEGF) production, and interleukin-6 expression, have been studied, but the exact mechanism of development of cerebral edema in meningioma patients remains unclear [4, 17, 21, 36]. Due to the blood-brain-barrier disruption, fluid is transferred to the extracellular space leading to disturbances of synaptic functioning and signal conduction across axons and dendrites [28]. White matter is particularly vulnerable to vasogenic edema, which tends to extend along neighboring fiber tracks. Furthermore, edema might lead to mass effect and thus to further compression of surrounding brain, sometimes resulting in local ischemia. These mechanisms may lead to cognitive deficits.
Cognitive deficits in brain tumor patients might also be attributed to surgical treatment, to the occurrence of seizures, to the treatment with antiepileptic drugs, or to the tumor itself [6, 11, 12]. Studies describing the association between tumor volume and the extent of peritumoral edema are inconsistent [2, 7, 10, 27]. In a previous study, we have shown that the addition of radiotherapy after surgery does not seem to have significant early (median follow up 3.3 years) detrimental impact on an already impaired cognitive functioning in meningioma patients [31]. We found positive associations between preoperative tumor volume and postoperative cognitive functioning. Furthermore, post hoc analysis yielded positive associations between the extent of preoperative edema and preoperative tumor volume. Although the association between preoperative edema and cognitive functioning was stronger than the association between preoperative tumor volume and cognitive functioning, tumor volume is a possible confounding factor for the association found between edema and cognitive performance. Regarding the mechanical effects exerted by both the tumor and the surrounding edema, it is most likely that the combination of these volumes might have negatively affected neurocognitive outcome. A relatively recent study also showed larger tumor volumes to be associated with poorer neurocognitive outcomes [14]. Considering the fact that surgery led to a significant reduction in tumor and edema mass (Table 2), we do not expect postoperative residual tumor and edema to affect cognition. Although our study shows that preoperative peritumoral edema is associated with limitations in cognitive functioning after surgery, this preoperative peritumoral edema does not seem to affect HRQOL. A recent systematic review by Najafabadi et al. describes both worse and better HRQOL scores in meningioma patients compared with healthy controls. Based on the available results, they conclude that, in general, meningioma patients have a clinically relevant worse HRQOL than healthy controls [19]. In our study, HRQOL in treated meningioma patients was comparable to that of the general population. A correlation between preoperative edema and HRQOL was not found. As far as we know, the effect of peritumoral edema and tumor volume on HRQOL has not been studied in meningioma patients before. The relatively unaffected HRQOL in our study may be explained by the fact that patients might have adapted to their postsurgical functioning or that a patient’s understanding or perception of HRQOL has changed over time, the so-called response shift [26]. Furthermore, Waagemans et al. showed that HRQOL particularly is compromised in meningioma patients with major cognitive deficits and those using AEDs [32].
Some shortcomings of this study need to be addressed. The number of patients is too small for subgroup analyses. The effect of epilepsy burden (i.e., AED use and seizure frequency), localization and lateralization of the tumor, and treatment-related factors on cognitive functioning and HRQOL could therefore not be studied. Pre- and postoperative amount of tumor may also influence cognition. Multivariate analysis to correct for tumor volume could not be performed, because of the small amount of patients.
Furthermore, since preoperative cognitive assessment is lacking, we could not analyze whether surgery itself also influences cognition. The effects of surgery on cognition in meningioma patients are not clear. Markovic et al. suggest that postoperative complications are more frequent in meningioma patients with peritumoral edema, which indicates the possibility of cognitive decline after surgery [18]. However, Tucha et al. indicate postoperative improvements in attentional functions in meningioma patients [29].
Although our results suggest that meningioma patients with larger volumes of preoperative peritumoral edema are at risk of experiencing longer-term cognitive deficits, future research efforts should focus on underlying causes and the question whether (early) reduction of peritumoral edema results in an improvement of cognition. Perhaps peritumoral edema in otherwise asymptomatic patients should prompt earlier surgery, or early treatment with medication (e.g., steroids, angiogenesis inhibitors) in an effort to prevent, or ameliorate, longer-term cognitive functioning. To meet this aim, prospective studies with larger patient groups including pre- and postoperative assessment are needed.
Conclusion
Our results suggest that meningioma patients with larger volumes of preoperative peritumoral edema may be at risk of experiencing limitations in longer-term cognitive functioning. This knowledge is useful for neurologists and neurosurgeons treating patients with a meningioma, but more studies regarding the effect of peritumoral edema on cognitive functioning in meningioma patients are necessary.
References
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E (1998) Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 51:1055–1068
Abe T, Black PM, Ojemann RG, Hedley-White ET (1994) Cerebral edema in intracranial meningiomas: evidence for local and diffuse patterns and factors associated with its occurrence. Surg Neurol 42:471–475
Benz LS, Wrensch MR, Schildkraut JM, Bondy ML, Warren JL, Wiemels JL, Claus EB (2018) Quality of life after surgery for intracranial meningioma. Cancer 124:161–166. https://doi.org/10.1002/cncr.30975
Bitzer M, Wockel L, Morgalla M, Keller C, Friese S, Heiss E, Meyermann R, Grote E, Voigt K (1997) Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochir 139:1136–1142
de Bie S (1987) Standard questions 1987: proposal for uniformization of questions regarding background variables and interviews [Standaardvragen 1987: Voorstellen voor uniformering van vraagstellingen naar achtergrondkenmerken en interviews], 2nd edn. Leiden University Press, Leiden
Dijkstra M, van Nieuwenhuizen D, Stalpers LJ, Wumkes M, Waagemans M, Vandertop WP, Heimans JJ, Leenstra S, Dirven CM, Reijneveld JC, Klein M (2009) Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry 80:910–915. https://doi.org/10.1136/jnnp.2007.138925
Gurkanlar D, Er U, Sanli M, Ozkan M, Sekerci Z (2005) Peritumoral brain edema in intracranial meningiomas. J Clin Neurosci 12:750–753. https://doi.org/10.1016/j.jocn.2004.09.029
Jolles J, van Boxtel MP, Ponds RW, Metsemakers JF, Houx PJ (1998) The Maastricht aging study (MAAS). The longitudinal perspective of cognitive aging. Tijdschr Gerontol Geriatr 29:120–129
Karnofsky DA (1948) The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer vol 1
Kim BW, Kim MS, Kim SW, Chang CH, Kim OL (2011) Peritumoral brain edema in meningiomas: correlation of radiologic and pathologic features. J Korean Neurosurg Soc 49:26–30. https://doi.org/10.3340/jkns.2011.49.1.26
Klein M, Engelberts NH, van der Ploeg HM, Kasteleijn-Nolst Trenite DG, Aaronson NK, Taphoorn MJ, Baaijen H, Vandertop WP, Muller M, Postma TJ, Heimans JJ (2003) Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol 54:514–520. https://doi.org/10.1002/ana.10712
Klein M, Heimans JJ, Aaronson NK, van der Ploeg HM, Grit J, Muller M, Postma TJ, Mooij JJ, Boerman RH, Beute GN, Ossenkoppele GJ, van Imhoff GW, Dekker AW, Jolles J, Slotman BJ, Struikmans H, Taphoorn MJ (2002) Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 360:1361–1368
Lee KJ, Joo WI, Rha HK, Park HK, Chough JK, Hong YK, Park CK (2008) Peritumoral brain edema in meningiomas: correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol 69:350–355; discussion 355. https://doi.org/10.1016/j.surneu.2007.03.027
Liouta E, Koutsarnakis C, Liakos F, Stranjalis G (2016) Effects of intracranial meningioma location, size, and surgery on neurocognitive functions: a 3-year prospective study. J Neurosurg 124:1578–1584. https://doi.org/10.3171/2015.6.JNS1549
Lobato RD, Alday R, Gomez PA, Rivas JJ, Dominguez J, Cabrera A, Madero S, Ayerbe J (1996) Brain oedema in patients with intracranial meningioma. Correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochir 138:485–493 discussion 493-484
Longstreth WT Jr, Dennis LK, McGuire VM, Drangsholt MT, Koepsell TD (1993) Epidemiology of intracranial meningioma. Cancer 72:639–648
Maiuri F, Gangemi M, Cirillo S, Delehaye L, Gallicchio B, Carandente M, Giamundo A (1987) Cerebral edema associated with meningiomas. Surg Neurol 27:64–68
Markovic M, Antunovic V, Milenkovic S, Zivkovic N (2013) Prognostic value of peritumoral edema and angiogenesis in intracranial meningioma surgery. J BUON 18:430–436
Najafabadi AHZ, Peeters MCM, Dirven L, Lobatto DJ, Groen JL, Broekman MLD, Peerdeman SM, Peul WC, Taphoorn MJB, van Furth WR (2017) Impaired health-related quality of life in meningioma patients-a systematic review. Neuro-Oncology 19:897–907. https://doi.org/10.1093/neuonc/now250
Order SE, Hellman S, Von Essen CF, Kligerman MM (1968) Improvement in quality of survival following whole-brain irradiation for brain metastasis. Radiology 91:149–153. https://doi.org/10.1148/91.1.149
Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y (2013) Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. J Neuro-Oncol 111:49–57. https://doi.org/10.1007/s11060-012-0989-y
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology 19:v1–v88. https://doi.org/10.1093/neuonc/nox158
Paek SH, Kim CY, Kim YY, Park IA, Kim MS, Kim DG, Jung HW (2002) Correlation of clinical and biological parameters with peritumoral edema in meningioma. J Neuro-Oncol 60:235–245
Radhakrishnan K, Mokri B, Parisi JE, O'Fallon WM, Sunku J, Kurland LT (1995) The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol 37:67–73. https://doi.org/10.1002/ana.410370113
Salpietro FM, Alafaci C, Lucerna S, Iacopino DG, Todaro C, Tomasello F (1994) Peritumoral edema in meningiomas: microsurgical observations of different brain tumor interfaces related to computed tomography. Neurosurgery 35:638–641 discussion 641-632
Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MA, Fayers PM (2006) The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res 15:1533–1550. https://doi.org/10.1007/s11136-006-0025-9
Simis A, Pires de Aguiar PH, Leite CC, Santana PA Jr, Rosemberg S, Teixeira MJ (2008) Peritumoral brain edema in benign meningiomas: correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg Neurol 70:471–477; discussion 477. https://doi.org/10.1016/j.surneu.2008.03.006
Steinvorth S, Welzel G, Fuss M, Debus J, Wildermuth S, Wannenmacher M, Wenz F (2003) Neuropsychological outcome after fractionated stereotactic radiotherapy (FSRT) for base of skull meningiomas: a prospective 1-year follow-up. Radiother Oncol 69:177–182
Tucha O, Smely C, Preier M, Becker G, Paul GM, Lange KW (2003) Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg 98:21–31. https://doi.org/10.3171/jns.2003.98.1.0021
van Alkemade H, de Leau M, Dieleman EM, Kardaun JW, van Os R, Vandertop WP, van Furth WR, Stalpers LJ (2012) Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 14:658–666. https://doi.org/10.1093/neuonc/nos013
van Nieuwenhuizen D, Klein M, Stalpers LJ, Leenstra S, Heimans JJ, Reijneveld JC (2007) Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neuro-Oncol 84:271–278. https://doi.org/10.1007/s11060-007-9366-7
Waagemans ML, van Nieuwenhuizen D, Dijkstra M, Wumkes M, Dirven CM, Leenstra S, Reijneveld JC, Klein M, Stalpers LJ (2011) Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery 69:72–78; discussion 78-79. https://doi.org/10.1227/NEU.0b013e318212badb
Wade DT, Collin C (1988) The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud 10:64–67
Whittle IR, Smith C, Navoo P, Collie D (2004) Meningiomas. Lancet 363:1535–1543. https://doi.org/10.1016/S0140-6736(04)16153-9
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neuro-Oncol 99:307–314. https://doi.org/10.1007/s11060-010-0386-3
Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K (1999) Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer 85:936–944
Acknowledgements
The authors thank Corine Lagemaat and Ewa Szymanska (VU University Medical Center, Amsterdam) for their valuable contributions to the project. Regarding the valuable comparisons with healthy controls, we are indebted to Jelle Jolles (Maastricht University) for use of the Maastricht Aging Study cognitive data set, and to Neil Aaronson (Netherlands Cancer Institute) for use of the Dutch SF-36 healthy population data set.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study has been evaluated and approved by the Medical Ethical Committee of the VU University Medical Center in Amsterdam. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Meningioma
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Nieuwenhuizen, D., Slot, K.M., Klein, M. et al. The association between preoperative edema and postoperative cognitive functioning and health-related quality of life in WHO grade I meningioma patients. Acta Neurochir 161, 579–588 (2019). https://doi.org/10.1007/s00701-019-03819-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03819-2