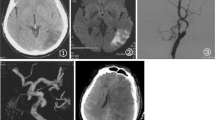
Abstract
Background
One of the major causes for performing unplanned re-exploration of a craniotomy after microsurgery for unruptured intracranial aneurysms (UIAs) is compromised distal blood flow after clipping. Therefore, it is important to identify the causes of compromised distal blood flow after clipping and the factors that influence the prognosis for re-exploration in order to decrease ischemic complications related to clipping UIAs.
Method
Between January 2007 and December 2013, 1954 patients underwent microsurgery for UIAs. In this cohort, 20 patients (1.0 %) required unplanned re-exploration of the craniotomy for several reasons, and 11 patients (0.6 %) underwent unplanned re-exploration with clip repositioning or changing of the previous clip because of compromised distal blood flow after clipping. Patient characteristics, aneurysm properties, intraoperative findings, annual incidence and prognosis were analyzed in these 11 patients.
Results
The annual incidence of re-exploration has gradually decreased since the introduction of several intraoperative monitoring techniques. In total, 3.0 % of UIAs in the M1 trunk, 0.8 % of UIAs at the origin of the anterior choroidal artery (AchA) and 0.5 % of UIAs at the bifurcation of the middle cerebral artery (MCA) required re-exploration. Here, all 11 UIAs had broad necks, and atherosclerosis was identified around 10 UIAs. Six patients with compromised MCA flow demonstrated relatively better outcomes following re-exploration than five patients with a compromised lenticulostriate artery (LSA) or AchA flow. Four patients with delayed ischemic symptoms demonstrated relatively better outcomes than the seven patients who developed ischemic symptoms immediately postoperatively.
Conclusion
Clinicians need to be more careful not to compromise distal blood flow when clipping UIAs at the MCA and AchA origin. Various intraoperative monitoring techniques can help reduce the incidence of compromised distal blood flow after clipping.



Similar content being viewed by others
References
Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S (2010) Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke 41:1471–1476
Bacigaluppi S, Fontanella M, Manninen P, Ducati A, Tredici G, Gentili F (2012) Monitoring techniques for prevention of procedure-related ischemic damage in aneurysm surgery. World Neurosurg 78:276–288
Barker FG 2nd, Amin-Hanjani S, Butler WE, Ogilvy CS, Carter BS (2003) In-hospital mortality and morbidity after surgical treatment of unruptured intracranial aneurysms in the United States, 1996–2000: the effect of hospital and surgeon volume. Neurosurgery 52:995–1007, discussion 1007–1009
Bhatia S, Sekula RF, Quigley MR, Williams R, Ku A (2011) Role of calcification in the outcomes of treated, unruptured, intracerebral aneurysms. Acta Neurochir (Wien) 153:905–911
Bohnstedt BN, Kemp WJ 3rd, Li Y, Payner TD, Horner TG, Leipzig TJ, Cohen-Gadol AA (2013) Surgical treatment of 127 anterior choroidal artery aneurysms: a cohort study of resultant ischemic complications. Neurosurgery 73:933–939, discussion 939–940
Brilstra EH, Rinkel GJ, van der Graaf Y, Sluzewski M, Groen RJ, Lo RT, Tulleken CA (2004) Quality of life after treatment of unruptured intracranial aneurysms by neurosurgical clipping or by embolisation with coils. A prospective, observational study. Cerebrovasc Dis 17:44–52
Cho MS, Kim MS, Chang CH, Kim SW, Kim SH, Choi BY (2008) Analysis of clip-induced ischemic complication of anterior choroidal artery aneurysms. J Korean Neurosurg Soc 43:131–134
Clatterbuck RE, Galler RM, Tamargo RJ, Chalif DJ (2006) Orthogonal interlocking tandem clipping technique for the reconstruction of complex middle cerebral artery aneurysms. Neurosurgery 59:ONS347–ONS351, discussion ONS351–352
Cui H, Wang Y, Yin Y, Wan J, Fei Z, Gao W, Jiang J (2011) Role of intraoperative microvascular Doppler in the microsurgical management of intracranial aneurysms. J Clin Ultrasound 39:27–31
Dashti R, Laakso A, Niemela M, Porras M, Hernesniemi J (2009) Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol 71:543–550, discussion 550
Dashti R, Rinne J, Hernesniemi J, Niemela M, Kivipelto L, Lehecka M, Karatas A, Avci E, Ishii K, Shen H, Pelaez JG, Albayrak BS, Ronkainen A, Koivisto T, Jaaskelainen JE (2007) Microneurosurgical management of proximal middle cerebral artery aneurysms. Surg Neurol 67:6–14
de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A (2008) Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery 62:1300–1310
Elsharkawy A, Lehecka M, Niemela M, Billon-Grand R, Lehto H, Kivisaari R, Hernesniemi J (2013) A new, more accurate classification of middle cerebral artery aneurysms: computed tomography angiographic study of 1,009 consecutive cases with 1,309 middle cerebral artery aneurysms. Neurosurgery 73:94–102, discussion 102
Elsharkawy A, Niemela M, Lehecka M, Lehto H, Jahromi BR, Goehre F, Kivisaari R, Hernesniemi J (2014) Focused opening of the sylvian fissure for microsurgical management of MCA aneurysms. Acta Neurochir (Wien) 156:17–25
Florence G, Guerit JM, Gueguen B (2004) Electroencephalography (EEG) and somatosensory evoked potentials (SEP) to prevent cerebral ischaemia in the operating room. Neurophysiol Clin 34:17–32
Gerlach R, Beck J, Setzer M, Vatter H, Berkefeld J, Mesnil D, de Rochemont R, Raabe A, Seifert V (2007) Treatment related morbidity of unruptured intracranial aneurysms: results of a prospective single centre series with an interdisciplinary approach over a 6 year period (1999–2005). J Neurol Neurosurg Psychiatry 78:864–871
Guo L, Gelb AW (2011) The use of motor evoked potential monitoring during cerebral aneurysm surgery to predict pure motor deficits due to subcortical ischemia. Clin Neurophysiol 122:648–655
Heiss WD (2011) The ischemic penumbra: correlates in imaging and implications for treatment of ischemic stroke. The johann Jacob Wepfer award 2011. Cerebrovasc Dis 32:307–320
Hosoda K, Fujita S, Kawaguchi T, Shose Y, Hamano S (1995) Saccular aneurysms of the proximal (M1) segment of the middle cerebral artery. Neurosurgery 36:441–446
Investigators ISoUIA (1998) Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med 339:1725–1733
Irie T, Yoshitani K, Ohnishi Y, Shinzawa M, Miura N, Kusaka Y, Miyazaki S, Miyamoto S (2010) The efficacy of motor-evoked potentials on cerebral aneurysm surgery and new-onset postoperative motor deficits. J Neurosurg Anesthesiol 22:247–251
Ishikawa T, Nakayama N, Moroi J, Kobayashi N, Kawai H, Muto T, Yasui N (2009) Concept of ideal closure line for clipping of middle cerebral artery aneurysms–technical note. Neurol Med Chir (Tokyo) 49:273–277, discussion 277–278
Kazumata K, Kamiyama H, Ishikawa T, Takizawa K, Maeda T, Makino K, Gotoh S (2003) Operative anatomy and classification of the sylvian veins for the distal transsylvian approach. Neurol Med Chir (Tokyo) 43:427–433, discussion 434
Kim BM, Kim DI, Shin YS, Chung EC, Kim DJ, Suh SH, Kim SY, Park SI, Choi CS, Won YS (2008) Clinical outcome and ischemic complication after treatment of anterior choroidal artery aneurysm: comparison between surgical clipping and endovascular coiling. AJNR Am J Neuroradiol 29:286–290
King JT Jr, Berlin JA, Flamm ES (1994) Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg 81:837–842
Krayenbuhl N, Erdem E, Oinas M, Krisht AF (2009) Symptomatic and silent ischemia associated with microsurgical clipping of intracranial aneurysms: evaluation with diffusion-weighted MRI. Stroke 40:129–133
Lee YS, Park J (2013) Anterior choroidal artery aneurysm surgery: ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc 54:86–92
Moroi J, Hadeishi H, Suzuki A, Yasui N (2005) Morbidity and mortality from surgical treatment of unruptured cerebral aneurysms at research institute for brain and blood vessels-akita. Neurosurgery 56:224–231, discussion 224–231
Neuloh G, Schramm J (2004) Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg 100:389–399
Niskanen M, Koivisto T, Rinne J, Ronkainen A, Pirskanen S, Saari T, Vanninen R (2005) Complications and postoperative care in patients undergoing treatment for unruptured intracranial aneurysms. J Neurosurg Anesthesiol 17:100–105
Nomura M, Kida S, Kita D, Hasegawa M, Matsui O, Yamashita J (2000) Anomalous origin of anterior choroidal artery associated with an aneurysm. Acta Neurochir (Wien) 142:1067–1068
Ohno K, Arai T, Isotani E, Nariai T, Hirakawa K (1999) Ischaemic complication following obliteration of unruptured cerebral aneurysms with atherosclerotic or calcified neck. Acta Neurochir (Wien) 141:699–705, discussion 705–696
Park DH, Kang SH, Lee JB, Lim DJ, Kwon TH, Chung YG, Lee HK (2008) Angiographic features, surgical management and outcomes of proximal middle cerebral artery aneurysms. Clin Neurol Neurosurg 110:544–551
Park W, Ahn JS, Park JC, Kwon Do H, Kwun BD, Kim CJ (2014) Re-exploration of the craniotomy after surgical treatment of unruptured intracranial aneurysms. Acta Neurochir (Wien) 156:869–877
Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF (2005) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103:982–989
Raaymakers TW, Rinkel GJ, Limburg M, Algra A (1998) Mortality and morbidity of surgery for unruptured intracranial aneurysms: a meta-analysis. Stroke 29:1531–1538
Rinne J, Hernesniemi J, Niskanen M, Vapalahti M (1996) Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery 38:2–11
Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M (1994) Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol 481(Pt 1):243–250
Sasaki T, Kodama N, Matsumoto M, Suzuki K, Konno Y, Sakuma J, Endo Y, Oinuma M (2007) Blood flow disturbance in perforating arteries attributable to aneurysm surgery. J Neurosurg 107:60–67
Seifert V, Gerlach R, Raabe A, Guresir E, Beck J, Szelenyi A, Setzer M, Vatter H, Mesnil D, de Rochemont R, Zanella F, Sitzer M, Berkefeld J (2008) The interdisciplinary treatment of unruptured intracranial aneurysms. Dtsch Arztebl Int 105:449–456
Shibata Y, Fujita S, Kawaguchi T, Hosoda K, Komatsu H, Tamaki N (2000) Use of microvascular Doppler sonography in aneurysm surgery on the anterior choroidal artery. Neurol Med Chir (Tokyo) 40:30–35, discussion 35–37
Suzuki K, Mikami T, Sugino T, Wanibuchi M, Miyamoto S, Hashimoto N, Mikuni N (2014) Discrepancy between voluntary movement and motor-evoked potentials in evaluation of motor function during clipping of anterior circulation aneurysms. World Neurosurg 82:e739–e745
Szelenyi A, Beck J, Strametz R, Blasel S, Oszvald A, Raabe A, Seifert V (2011) Is the surgical repair of unruptured atherosclerotic aneurysms at a higher risk of intraoperative ischemia? Clin Neurol Neurosurg 113:129–135
Szelenyi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V (2006) Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: intraoperative changes and postoperative outcome. J Neurosurg 105:675–681
Ture U, Yasargil MG, Al-Mefty O, Yasargil DC (2000) Arteries of the insula. J Neurosurg 92:676–687
Umansky F, Gomes FB, Dujovny M, Diaz FG, Ausman JI, Mirchandani HG, Berman SK (1985) The perforating branches of the middle cerebral artery. A microanatomical study. J Neurosurg 62:261–268
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Yang I, Lawton MT (2008) Clipping of complex aneurysms with fenestration tubes: application and assessment of three types of clip techniques. Neurosurgery 62:ONS371–ONS378, discussion 378–379
Conflict of interest
All authors listed on the title page certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Among their impressive series of operated unruptured intracranial aneurysms, the authors present 11 cases that were re-explored because of compromised distal blood flow immediately postoperatively. Although without any doubt these represent negative incidents, they were managed promptly, and most cases eventually had a favorable outcome.
In unruptured aneurysm surgery, local brain conditions are usually calm and can mislead the surgeon following an uneventful clip placement. Intraoperative techniques such as angiography and neurophysiology are extremely important and should be an integral part of aneurysm surgery in particular when dealing with middle cerebral artery aneurysms. Additionally, largely dependent on the surgeon’s judgment and experience, correct clip positioning, placement of multiple clips, or opening plus resection of the aneurysm sac especially for large middle cerebral aneurysms can help avoid such events.
Even in experienced hands and in large centers, compromised blood flow immediately after aneurysm surgery is still a reality and can be easily missed. Special emphasis should be placed on the prevention of such events. Restricted flow in anterior choroidal and lenticulostriate arteries can frequently lead to irreversible neurologic sequelae and is associated with worse prognosis. Besides surgeon’s skills and experience, the implementation of more advanced intraoperative neuroimaging techniques will likely further reduce the need for reoperation and improve outcomes.
Parmenion Tsitsopoulos
Uppsala, Sweden
When planning clipping of an unruptured cerebral aneurysm, the neck length and potential calcifications must be analyzed beforehand to help in choosing the right clip(s) and informing the scrub nurse. The length of the clip blades should be a minimum of 1.5 times the length of the neck for perfect fit. Too short a blade (1) may lead to filling of the aneurysm, and too long a blade may cause inadvertent kinking of the parent artery and closure of the adjacent arteries with ischemic sequelae. When calcifications are in the neck, a stronger (= longer) clip may be needed or preferably more than one with the optimal length to avoid clip slipping—this may cause either compromised blood flow, like in the present series, or filling of the neck. Also, using temporary clips before clipping and puncturing of the aneurysm after clipping to soften it for less pressure and resistance of the neck facilitates proper and permanent positioning of the blades. In giant calcified aneurysms, a vascular clamp may be used to assist clipping (2). After clipping and observation under the operative microscope, ICG angiography (3) and Doppler US will be performed together with flow measurements if possible followed by CT angiography the next day at our department or DSA in case of complex giant aneurysms. In case of neurological deficits after waking up the patient, these studies should be performed immediately to allow quick repositioning of the clip when indicated.
1. Celik O, Niemelä M, Romani R, Hernesniemi J: Inappropriate application of Yasargil aneurysm clips: a new observation and technical remark. Neurosurgery 66:84–87, 2010
2. Navratil O, Lehecka M, Lehto H, Dashti R, Kivisaari R, Niemelä M, Hernesniemi JA: Vascular clamp-assisted clipping of thick-walled giant aneurysms. Neurosurgery 64:113–20, 2009
3. Dashti R, Laakso A, Niemelä M, Porras M, Hernesniemi J. Microscope-integrated ner-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol 71 (5):543–50, 2009
Mika Niemelä
Helsinki,Finland
Rights and permissions
About this article
Cite this article
Park, W., Ahn, J.S., Lee, S.H. et al. Results of re-exploration because of compromised distal blood flow after clipping unruptured intracranial aneurysms. Acta Neurochir 157, 1015–1024 (2015). https://doi.org/10.1007/s00701-015-2408-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2408-6