Abstract
Background
Postoperative hyperperfusion is a potential complication of the direct bypass procedure for moyamoya disease (MMD). However, no reliable modality is available yet for the prediction of postoperative hyperperfusion during surgery for MMD. This study aimed to investigate whether semiquantitative analysis of indocyanine green (ICG) videoangiography could contribute to the prediction of postoperative hyperperfusion in MMD.
Methods
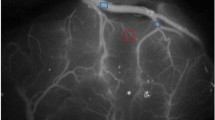
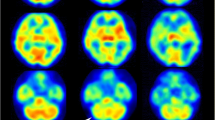
This study included 12 hemispheres from 10 patients who underwent surgical revascularization for MMD. Intraoperative ICG videoangiography was performed before and after a direct bypass procedure. The ICG intensity-time curves were semiquantitatively analyzed to evaluate cortical perfusion by calculating the blood flow index (BFI). Using single-photon emission computed tomography (SPECT), postoperative cerebral blood flow measurements were performed thrice: immediately, and 2 and 7 days after surgery.
Results
BFI significantly increased from 21.3 ± 10.5 to 38.4 ± 20.0 after bypass procedures in all the hemispheres (p < 0.01). The ratio of BFI before and after the bypass procedure was 2.4 ± 2.0, ranging from 0.5 to 8.0. Postoperative hyperperfusion was observed in nine of the 12 operated hemispheres within 7 days after surgery. Of these, three hemispheres developed hyperperfusion immediately after surgery. In the adult cases, the increase in the ratio of BFI after bypass was significantly greater in those who developed hyperperfusion immediately after surgery than in those who did not (6.5 ± 0.5 vs. 1.8 ± 2.1, p < 0.01). In contrast, no significant increase in BFI was observed in the pediatric MMD patients who experienced immediate hyperperfusion. No correlation between the changes in BFI and the occurrence of delayed hyperperfusion was observed.
Conclusions
Our results suggest that semiquantitative analysis of BFI by intraoperative ICG videoangiography is useful in evaluating changes in cortical perfusion after bypass procedures for MMD and can predict the occurrence of early-onset hyperperfusion in MMD patients after direct bypass.




Similar content being viewed by others
References
Kuroda S, Houkin K (2008) Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7:1056–1066
Suzuki J, Takaku A (1969) Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20:288–299
Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do HM, Marks MP, Steinberg GK (2009) Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg 111:927–935
Houkin K, Kamiyama H, Takahashi A, Kuroda S, Abe H (1997) Combined revascularization surgery for childhood moyamoya disease: STA-MCA and encephalo-duro-arterio-myo-synangiosis. Childs Nerv Syst 13:24–29
Ishikawa T, Houkin K, Kamiyama H, Abe H (1997) Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke 28:1170–1173
Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H (1992) Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg 77:84–89
Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y (2010) Novel bypass surgery for moyamoya disease using pericranial flap: its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery 66:1093–1101, discussion 1101
Miyamoto S, Akiyama Y, Nagata I, Karasawa J, Nozaki K, Hashimoto N, Kikuchi H (1998) Long-term outcome after STA-MCA anastomosis for moyamoya disease. Neurosurg Focus 5:e5
Kuroda S, Houkin K (2012) Bypass surgery for moyamoya disease: concept and essence of sugical techniques. Neurol Med Chir (Tokyo) 52:287–294
Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T (2007) Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol 67:273–282
Ogasawara K, Komoribayashi N, Kobayashi M, Fukuda T, Inoue T, Yamadate K, Ogawa A (2005) Neural damage caused by cerebral hyperperfusion after arterial bypass surgery in a patient with moyamoya disease: case report. Neurosurgery 56:E1380, discussion E1380
Sasamori T, Kuroda S, Nakayama N, Iwasaki Y (2010) Incidence and pathogenesis of transient cheiro-oral syndrome after surgical revascularization for moyamoya disease. Neurosurgery 67:1054–1059, discussion 1060
Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S (1998) Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease–case report. Neurol Med Chir (Tokyo) 38:420–424
Furuya K, Kawahara N, Morita A, Momose T, Aoki S, Kirino T (2004) Focal hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease. Case report. J Neurosurg 100:128–132
Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T (2008) Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 69:281–286, discussion 286–287
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T (2009) Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71:442–447
Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N (2012) Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke 43:2610–2616
Killory BD, Nakaji P, Gonzales LF, Ponce FA, Wait SD, Spetzler RF (2009) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery 65:456–462, discussion 462
Kuroiwa T, Kajimoto Y, Ohta T (2001) Development and clinical application of near-infrared surgical microscope: preliminary report. Minim Invasive Neurosurg 44:240–242
Pena-Tapia PG, Kemmling A, Czabanka M, Vajkoczy P, Schmiedek P (2008) Identification of the optimal cortical target point for extracranial-intracranial bypass surgery in patients with hemodynamic cerebrovascular insufficiency. J Neurosurg 108:655–661
Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V (2003) Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52:132–139, discussion 139
Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF (2005) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103:982–989
Woitzik J, Horn P, Vajkoczy P, Schmiedek P (2005) Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 102:692–698
Uchino H, Nakamura T, Houkin K, Murata J, Saito H, Kuroda S (2013) Semiquantitative analysis of indocyanine green videoangiography for cortical perfusion assessment in superficial temporal artery to middle cerebral artery anastomosis. Acta Neurochir (Wien) 155:599–605
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52:245–266
Hokari M, Kuroda S, Shiga T, Nakayama N, Tamaki N, Iwasaki Y (2008) Combination of a mean transit time measurement with an acetazolamide test increases predictive power to identify elevated oxygen extraction fraction in occlusive carotid artery diseases. J Nucl Med 49:1922–1927
Hokari M, Kuroda S, Shiga T, Nakayama N, Tamaki N, Iwasaki Y (2009) Impact of oxygen extraction fraction on long-term prognosis in patients with reduced blood flow and vasoreactivity because of occlusive carotid artery disease. Surg Neurol 71:532–538, discussion 538, 538–539
Kuroda S, Shiga T, Houkin K, Ishikawa T, Katoh C, Tamaki N, Iwasaki Y (2006) Cerebral oxygen metabolism and neuronal integrity in patients with impaired vasoreactivity attributable to occlusive carotid artery disease. Stroke 37:393–398
Kuroda S, Shiga T, Ishikawa T, Houkin K, Narita T, Katoh C, Tamaki N, Iwasaki Y (2004) Reduced blood flow and preserved vasoreactivity characterize oxygen hypometabolism due to incomplete infarction in occlusive carotid artery diseases. J Nucl Med 45:943–949
Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H (2001) Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 32:2110–2116
Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K (1993) Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 32:912–918, discussion 918–919
Kazumata K, Ito M, Tokairin K, Ito Y, Houkin K, Nakayama N, Kuroda S, Ishikawa T, Kamiyama H (2014) The frequency of postoperative stroke in moyamoya disease following combined revascularization: a single-university series and systematic review. J Neurosurg
Kuebler WM, Sckell A, Habler O, Kleen M, Kuhnle GE, Welte M, Messmer K, Goetz AE (1998) Noninvasive measurement of regional cerebral blood flow by near-infrared spectroscopy and indocyanine green. J Cereb Blood Flow Metab 18:445–456
Perbeck L, Lewis DH, Thulin L, Tyden G (1985) Correlation between fluorescein flowmetry, 133Xenon clearance and electromagnetic flow measurement: a study in the intestine of the pig. Clin Physiol 5:293–299
Woitzik J, Pena-Tapia PG, Schneider UC, Vajkoczy P, Thome C (2006) Cortical perfusion measurement by indocyanine-green videoangiography in patients undergoing hemicraniectomy for malignant stroke. Stroke 37:1549–1551
Hatazawa J, Fujita H, Kanno I, Satoh T, Iida H, Miura S, Murakami M, Okudera T, Inugami A, Ogawa T, Shimosegawa E, Noguchi K, Shohji Y, Uemura K (1995) Regional cerebral blood flow, blood volume, oxygen extraction fraction, and oxygen utilization rate in normal volunteers measured by the autoradiographic technique and the single breath inhalation method. Ann Nucl Med 9:15–21
Fujimura M, Inoue T, Shimizu H, Saito A, Mugikura S, Tominaga T (2012) Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis 33:436–445
Kawamata T, Kawashima A, Yamaguchi K, Hori T, Okada Y (2011) Usefulness of intraoperative laser Doppler flowmetry and thermography to predict a risk of postoperative hyperperfusion after superficial temporal artery-middle cerebral artery bypass for moyamoya disease. Neurosurg Rev 34:355–362, discussion 362
Kawamata T, Okada Y, Kawashima A, Yoneyama T, Yamaguchi K, Ono Y, Hori T (2009) Postcarotid endarterectomy cerebral hyperperfusion can be prevented by minimizing intraoperative cerebral ischemia and strict postoperative blood pressure control under continuous sedation. Neurosurgery 64:447–453, discussion 453–444
van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, de Leeuw PW (2005) Cerebral hyperperfusion syndrome. Lancet Neurol 4:877–888
Horie N, Fukuda Y, Izumo T, Hayashi K, Suyama K, Nagata I (2014) Indocyanine green videoangiography for assessment of postoperative hyperperfusion in moyamoya disease. Acta Neurochir (Wien) 156:919–926
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchino, H., Kazumata, K., Ito, M. et al. Intraoperative assessment of cortical perfusion by indocyanine green videoangiography in surgical revascularization for moyamoya disease. Acta Neurochir 156, 1753–1760 (2014). https://doi.org/10.1007/s00701-014-2161-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2161-2