Abstract
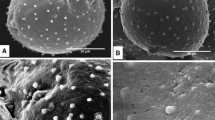
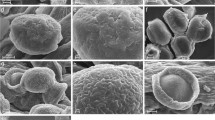
The morphology and ultrastructure of fresh pollen from nine species, one including two varieties representing seven genera of Annonaceae are described based on observations with scanning and transmission electron microscopy. The pollen grains are elliptic with a single furrow, or disulculate. Some are globose with no visible aperture or any indication of a pole. Ornamentation is smooth, rugulate, echinate or verrucate. The tectum is usually continuous and of the same thickness over the whole grain except for the aperture zone, where the exine elements are very often imperceptible. The infratectum may be granular, or columellae and granules are mixed together. The foot layer consists of continuous or irregularly contorted foliations. The endexine is distinct and thin, and varies slightly in thickness in some species, but is vaguely distinguishable in others. The intine is two-layered and consists of an entexine with many vesicular-fibrillar components with tubular extensions, and a more homogeneous endintine. The controversy around the presence of an endexine in Annonaceae is discussed, but whether its presence is ancestral cannot be determined. Data on fresh pollen are compared with those from similar studies on dried pollen.







Similar content being viewed by others
References
Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Brenner GJ (1996) Evidence for the earliest stage of angiosperm pollen evolution: a paleoequatorial section from Israel. In: Taylor DW, Hickey LJ (eds) Flowering plant origin, evolution and phylogeny. Chapman & Hall, New York, pp 91–115
Chaowasku T, Mols J, Van der Ham RWJM (2008) Pollen morphology of Miliusa and relatives (Annonaceae). Grana 47:175–184
Chatrou LW, Rainer H, Mass JM (2004) Annonaceae. In: Smith N, Mori SA, Henderson A, Stevenson DW, Heald SV (eds) Flowering plants of the neotropics. Princeton University Press, Princeton, pp 18–20
Couvreur TLP, Botermans M, Van Heuven BJ, Van der Ham RWJM (2008) Pollen morphology within the Monodora clade, a diverse group of five African Annonaceae genera. Grana 47:185–210
Doyle JA (2005) Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana 44:227–251
Doyle JA, Le Thomas A (1994) Cladistic analysis and pollen evolution in Annonaceae. Acta Bot Gallica 141:149–170
Doyle JA, Le Thomas A (2012) Evolution and phylogenetic significance of pollen in Annonaceae. Bot J Linn Soc 169:190–221
Doyle JA, Van Campo M, Lugardon B (1975) Observations on exine structure of Eucommiidites and lower cretaceous angiosperm pollen. Pollen Spores 17:429–484
Doyle JA, Bygrave P, le Thomas A (2000) Implications of molecular data for pollen evolution in Annonaceae. In: Harley MM, Morton CM, Blackmore S (eds) Pollen and spores: morphology and biology. Royal Botanic Gardens, Kew, pp 259–284
Gabarayeva NI (1993) Sporoderm development in Asimina triloba (Annonaceae). II. The development events after callose dissolution. Grana 32:210–220
Gabarayeva NI (1995) Pollen wall and tapetum development in Anaxagorea brevipes (Annonaceae): sporoderm substructure, cytoskeleton, sporopollenin precursor particles, and the endexine problem. Rev Palaeobot Palynol 85:123–152
Halbritter H, Hesse M (2004) Principal modes of infoldings in tricolp(or)ate Angiosperm pollen. Grana 43:1–14
Hesse M, Waha M (1989) A new look at the acetolysis method. Plant Syst Evol 163:147–152
Hesse M, Morawetz W, Ehrendorfer F (1985) Pollen ultrastructure and systematic affinities of Anaxagorea (Annonaceae). Plant Syst Evol 148:253–285
Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S (2009) Pollen terminology. An illustrated handbook. Springer, New York
Katifori E, Alben S, Cerda E, Nelson DR, Dumais J (2010) Foldable structures and the natural design of pollen grains. Proc Natl Acad Sci U S A 107:7635–7639
Kessler PJA (1993) Annonaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2., Flowering plants. Dicotyledons. Springer, Berlin, pp 93–129
Le Thomas A (1980) Ultrastructural characters of the pollen grains of African Annonaceae and their significance for the phylogeny of primitive angiosperms (first part). Pollen Spores 22:267–342
Le Thomas A (1981) Ultrastructural characters of the pollen grains of African Annonaceae and their significance for the phylogeny of primitive angiosperms (second part). Pollen Spores 23:5–36
Le Thomas A, Lugardon B (1972) Sur la structure fine des tétrades de deux Annonacées (Asteranthe asterias et Hexalobus monopetalus). C R Acad Sc Paris Sér D 275:1749–1752
Le Thomas A, Lugardon B (1976) Structure exinique chez quelques genres d’Annonaces. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine., Linnean Society Symposium series 1. Academic Press, London, pp 309–325
Le Thomas A, Lugardon B, Doyle JA (1994) Pollen ultrastructure and relationships of Fusaea (Baillon) Safford and Duguetia A. Saint-Hilaire (Annonaceae). Rev Palaeobot Palynol 83:55–64
Lora J, Testillano PS, Risueno MC, Hormaza JI, Herrero M (2009) Pollen development in Annona cherimola Mill. (Annonaceae). Implications for the evolution of aggregated pollen. BMC Plant Bio 9:129
Lugardon B, Le Thomas A (1974) Foliated structure of basal layer of ectexine in various Annonaceae. C R Acad Sci Paris Sér D 279:255–258
Mols JB, Co DLV, Gravendeel B, Chatrou LW, Pirie MD, van der Ham RWJM, van Marle EJ, Kessler PJA (2004) Morphological character evolution in the miliusoid clade (Annonaceae). In: Mols JB (ed) From Miliusa to Miliuseae to Miliusoid: identifying clades in Asian Annonaceae. Nationaal Herbarium Nederland, Leiden, pp 37–75
Punt W, Hoen PP, Blackmore S, Nilsson S, le Thomas A (2007) Glossary of pollen and spore terminology. Rev Palaeobot Palynol 143:1–81
Remizowa MV, Sokoloff DD, Macfarlane TD, Yadav SR, Prychid CJ, Rudall PJ (2008) Comparative pollen morphology in the early-divergent angiosperm family Hydatellaceae reveals variation at the infraspecific level. Grana 47:81–100
Sampson FB (2000) Pollen diversity in some modern Magnoliids. Int J Plant Sci 161:S193–S210
Sauquet H, Doyle JA, Scharaschkin T, Borsch T, Hilu KW, Chatrou LW, le Thomas A (2003) Phylogenetic analysis of Magnoliales and Myristicaceae based on multiple data sets: implications for character evolution. Bot J Linn Soc 142:125–186
Su YCF, Saunders RMK (2003) Pollen structure, tetrad cohesion and pollen-connecting threads in Pseuduvaria (Annonaceae). Bot J Linn Soc 143:69–78
Tsou C-H, Johnson DM (2003) Comparative development of aseptate and septate anthers of Annonaceae. Am J Bot 90:832–848
Waha M (1987) Different origins of fragile exines within the Annonaceae. Plant Syst Evol 158:23–27
Waha M, Hesse M (1988) Aperture types within Sapranthus and Polyalthia. Plant Syst Evol 161:135–146
Waha M, Morawetz W (1988) Pollen evolution and systematics in Annonaceae with special reference to the disulcate Australian endemic genera. Plant Syst Evol 161:1–12
Walker JW (1971a) Pollen morphology, phytogeography, and phylogeny of the Annonaceae. Contr Gray Herb 202:1–131
Walker JW (1971b) Elucidation of exine structure and sculpturing in the Annonaceae through combined use of light and scanning electron microscope. Pollen Spores 13:187–198
Walker JW (1971c) Contribution to the pollen morphology and phylogeny of the Annonaceae I. Grana 11:45–54
Walker JW (1976) Evolutionary significance of the exine in the pollen of primitive angiosperms. In: Ferguson IK, Müller J (eds) The evolutionary significance of the exine. Academic Press, London, pp 251–308
Walker JW, Skvarla JJ (1975) Primitively columellaless pollen: a new concept in the evolutionary morphology of angiosperms. Science 187:445–447
Walker JW, Walker AG (1984) Ultrastructure of lower cretaceous angiosperm pollen and the origin and early evolution of flowering plants. Ann Mo Bot Gard 71:464–521
Weber M, Ulrich S (2010) The endexine: a frequently overlooked pollen wall layer and a simple method for detection. Grana 49:83–90
Xu FX, Kirchoff BK (2008) Pollen morphology and ultrastructure of selected species of Annonaceae. Rev Palaeobot Palynol 150:140–153
Xue B, Su YCF, Mols JB, Kessler PJA, Saunders RMK (2011) Further fragmentation of the polyphyletic genus Polyalthia (Annonaceae): molecular phylogenetic support for a broader delimitation of Marsypopetalum. Syst Biod 9:17–26
Acknowledgments
This study was financially supported by the National Sciences Foundation of China (grant number 31270227, 30770140), the Knowledge Innovation Program of the Chinese Academy of Sciences (grant number KSCX2-EW-J-28) and the Key Laboratory of Plant Resources Conservation and Sustainable Utilization. The authors thank Mrs. Xiaoying Hu for her technical assistance with the SEM and Mrs. Xinlan Xu for assistance with TEM at the South China Botanical Garden, and also Mr. Qiang Wei for collecting samples. An anonymous reviewer and Jim Doyle are acknowledged for their helpful comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, F., de Craene, L.P.R. Pollen morphology and ultrastructure of selected species from Annonaceae. Plant Syst Evol 299, 11–24 (2013). https://doi.org/10.1007/s00606-012-0698-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-012-0698-1