Abstract
The aim of this study is verification of the taxonomic usefulness of the pollen grain features studied, based on pollen morphology of 32 wild species from all 4 subgenera and all 10 sections of the genus Rosa, mainly for delimitation of subgenera, sections, and species. The measurements and observations were carried out with both light microscopy and scanning electron microscopy. Only correctly formed pollen grains (30 per specimen) were measured, and 960 pollen grains were examined in total. They were analyzed for 14 quantitative features of pollen grains and exine sculpturing and the following qualitative ones: outline, shape, and operculum structure. Our study revealed that the diagnostic features of pollen grains studied were: exine sculpture, length of polar axis, and pollen shape (P/E ratio). On the basis of the above characters, five species were isolated and the remaining ones were included in several groups isolated on the basis of exine sculpture types. The following three exine sculpture types occurred in the species studied: granular-verrucate (in R. stellata), striate-psilate (in R. multibracteata and R. multiflora), and striate (the remaining species). R. banksiae is characterized by small pollen grains, while R. setigera has strongly elongated pollen with P/E ratio >1.5. Exine sculpture features considered to be diagnostic should be treated as auxiliary because they fail to differentiate individual species, although they can be helpful in distinguishing groups of species of similar exine sculpture. The arrangement of the species examined on a dendrogram only slightly corroborates division of the Rosa genus into subgenera and sections currently adopted in taxonomy (Rehder 1940). An interesting result was reported for the species studied from the Caninae (R. agrestis, R. canina, R. dumalis, R. jundzillii, and R. rubiginosa) section which, despite hybrid nature, with the exception of R. villosa, grouped in the same, most separated group of species.
Similar content being viewed by others
Introduction
The genus Rosa L. is distributed in the northern hemisphere in Europe, Asia, Ethiopia, the Middle East, and North America and contains, depending on the taxonomic approach adopted, 100–120, or even 250 species (Hutchinson 1964; Zieliński 1987; Nilsson 1997; Henker 2000; Kalkman 2004).
In modern rose taxonomy (i.e., excluding classification of hybrids), the genus Rosa is divided into four subgenera: Platyrhodon (R. roxburghii), Hulthemia (R. persica), Hesperrhodos (R. minutifolia and R. stellata), and Eurosa, which are separated on the basis of significant differences in fruit structure. The first three subgenera are monotypic (each representing a single species) or consist of two species, while the subgenus Eurosa contains all the remaining species (Rehder 1940, 1949). This subgenus is subdivided into 10 sections: Banksianae, Bracteatae, Caninae, Carolinae, Gallicanae, Indicae, Laevigatae, Pimpinellifoliae, Rosa (syn. sect. Cinnamomeae), and Synstylae. In this study, following the proposal that the type species for the section is R. cinnamomeae L., we use the section name Rosa and not Cinnamomeae (Cassiorhodon) (Rowley 1976; Nicolson 1992).
The particular species from the genus Rosa produce radially symmetric isopolar monads. Pollen are 3-colporate, rarely 4-colporate. Fusiform ectocolpi are slits, mostly constricted at the equator by arching sexinal, narrow pore flaps or an equatorial bridge. Pollen are with or without colpus opercula. Endoapertures are well developed, spheroidal or elliptic, and usually lie in the central part of colpus. Exine sculpturing is more often striate, very rarely verrucate. If striate, then parallel striae and grooves containing perforations predominate. In Rosa, perforations are large and often extend onto tectal striae (Reitsma 1966; Eide 1981; Hebda and Chinnappa 1990, 1994; Wrońska-Pilarek 2010; Bai et al. 2011).
Many palynologists believe that exine sculpture features are diagnostic at the level of both genus and species. The most important feature of exine sculpture are number and width of striae and grooves, as well as number, area, and size of perforations. Some scientists have emphasized that, for species delimitation within the genus Rosa, pollen shape, length of equatorial and polar axes, length of colpi, operculum structure, as well as presence or lack of costae colpi are very important (Reitsma 1966; Fogle 1977; Eide 1981; Matsuta et al. 1982; Marcucci et al. 1984; Hebda and Chinnappa 1990, 1994; Ueda 1992; Ueda and Okada 1994; Popek 1996; Shinwari and Khan 2004; Wrońska-Pilarek 2010).
Despite numerous publications, our knowledge about rose pollen grain structure is far from complete because the available descriptions are usually brief and, sometimes, are limited to giving mean dimensions, or researchers analyze individual, most important pollen grain characters (usually, exine structure); alternatively, only some selected species are characterized. Therefore, the aim of our study is verification of the taxonomic usefulness of quantitative and qualitative features of pollen grains, based on pollen morphology of 32 species from all subgenera and sections of the genus Rosa, from LM (light microscope) and SEM (scanning electron microscope) observations, mainly for delimitation of subgenera, sections, and the particular taxa.
Materials and methods
The study was conducted on 32 Rosa species representing all 4 subgenera and 10 sections of the genus Rosa. A list of the species analyzed with their affiliation to particular subgenera and sections is shown in Table 1.
The terminology follows Punt et al. (2007) and Hesse et al. (2009). Definitions of the palynological terms used in this article (Punt et al. 2007) can be found on the website http://www3.bio.uu.nl/palaeo/glossary/index.htm. The term “striae” is ambiguous in the palynological literature, thus we use it according to Hesse et al. (2009).
Pollen samples of 32 wild species of Rosa were collected in the Herbarium of the University of Vienna (27 species) and from natural sites in Poland and Slovakia (5 species; Table 1). Several randomly selected flowers were collected from each individual (rose bush). All plant specimens which supplied material for our studies were also deposited in the herbarium of the Department of Forestry Natural Foundations (POZNF), Poznan University of Life Sciences (Poland).
All samples were acetolyzed according to the method described by Wrońska-Pilarek (1998).
The observations were carried out with both light microscopy (Biolar 2308, Nikon HFX-DX) and scanning electron microscopy (ISI 60, Hitachi S-3000N).
The number of pollen grains analyzed provided statistically reliable data for estimating interspecific morphological variability of pollen grains (Wrońska-Pilarek and Jagodziński 2009). Only correctly formed pollen grains (30 per specimen) were measured, and 960 pollen grains were examined in total. They were analyzed for 14 quantitative features of pollen grains and exine sculpturing (Table 2) and the following qualitative ones: outline, shape, and “opercula” structure. Exine sculpture elements were measured on an area of 25 μm2 in accordance with the methods of Ueda and Tomita (1989).
The structures described as “opercula” refer to a distinctly delimited sexine/ectexine structure which covers part of an ectoaperture and which is completely isolated from the rest of the sexine (Wodehouse 1935); there can be, in some cases, no real opercula, but bulges of the intine at the beginning of pollen tube germination. Despite the above reservations, the term “operculum” is employed in this study as it is used by all palynologists who have described this structure so far (Teppner 1965; Reitsma 1966; Eide 1981; Faegri and Iversen 1989; Gonzalez Romano and Candau 1989; Hebda and Chinnappa 1990, 1994; Moore et al. 1991; Popek 1996; Zhou et al. 1999).
For each pollen grain feature, one-factor analysis of variance (ANOVA) was used to examine differences in mean values among subgenera, sections, and all species studied. When critical differences were noted, multiple comparisons were carried out using Tukey’s test for equal sample sizes. To show similarities and differences among the taxa studied, Ward’s hierarchical clustering method was used to compute closer groups based on all pollen grain morphological features. Statistical analyses were performed using JMP 8.0 (SAS Institute Inc., Cary, NC. USA; http://www.sas.com/).
Results
General pollen morphological description
A description of the pollen grain morphology of Rosa species studied is given below and illustrated with a few SEM photographs (Figs. 1–19). The morphological observations for quantitative features of exine sculpture are summarized in Table 3 and for remaining quantitative features of pollen grains in Table 4.
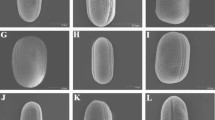
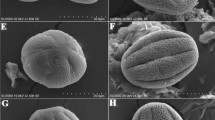
SEM micrographs of pollen grains of Rosa species studied. Figs. 1, 2. R. persica, R. gymnocarpa—pollen grains in equatorial view with two colpi. Figs. 3, 4. R. carolina, R. stellata—pollen grains in polar view with three colpi. Fig. 5. R. sericea—colporus with “opened” endoaperture, that is, endoaperture in which after acetolysis the membrane is gone. Fig. 6. R. multiflora—equatorial bridge formed by two bulges of ectexine (±of the same length) that meet in the middle of the colpus. Figs. 7–9. R. sericea, R. pendulina, R. multiflora—operculum wide and convex. Figs. 10, 11. R. bracteata, R. pendulina—operculum narrow, elongated, and flat. Figs. 12–19. Exine sculpture types according to classification of Ueda and Tomita (1989). Fig. 12. R. persica—IA type. Fig. 13. R. sericea—IB type. Fig. 14. R. gallica—IIA type. Fig. 15. R. pendulina—IIB type. Fig. 16. R. virginiana—IIIA type. Fig. 17. R. bracteata—IIIB type. Fig. 18. R. multiflora—V type. Fig. 19. R. stellata—VI type
The grains of Rosa species studied were 3 (4)-zonocolporate (Figs. 1–4). A majority of pollen grains, according to Erdtman’s (1952) pollen size classification, were medium (25.1–50 μm; 92.7%), rarely small (10–25 μm; 7.3%).
The average length of the polar axis (P) was 30.93 (range 20–48) μm. Generally speaking, the smallest mean P values were found for the pollen of R. banksiae (21.3 μm), R. stellata (24.3 μm), and R. nitida (26.5 μm), and the largest for R. multibracteata (36.5 μm) and R. moyesii (37.8 μm) (Table 4). A majority of small pollen occurred in the R. banksiae sample (all measured pollen grains were small at a very narrow range of axis P length; 20–24 μm), in R. stellata (73.3% of measured pollen; at length range of P 22-28 μm), and in R. nitida (30%; at length range of P 24–30 μm). A few small pollen grains (from one to four small grains, i.e., from 3.3% to 13.3%) occurred in five other species examined (R. blanda, R. villosa, R. laevigata, R. sertata, and R. multiflora). On the other hand, the longest pollen grains (with polar axis P ≥ 40 μm) were found in R. moyesii (40%; in the range 34–48 μm) and in R. multibracteata (16.7%; in the range 30–42 μm). Such large pollen grains, singly, were also found in R. persica and R. agrestis (Table 4).
The mean length of the equatorial diameter (E) was 26.09 (range 14–38) μm. Generally speaking, the shortest mean equatorial diameter occurred in the pollen of R. banksiae (17.6 μm), while the longest was in R. moyesii (30.2 μm; Table 4).
Outline in polar view was mostly circular or triangular with obtuse apices, more rarely elliptic, whereas in equatorial view the outline was mostly elliptic, rarely circular (Figs. 1–4).
The mean P/E ratio was 1.2, and ranged from 0.85 in R. stellata to 2.11 in R. setigera (Table 4). Pollen grains of the species examined were most frequently subprolate (41.7%), prolate-spheroidal (27.2%), and prolate (16.4%), rarely spheroidal (9.8%) and oblate-spheroidal (4.8%), and very rarely perprolate (0.1%) (Table 5). The highest number of subprolate pollen grains occurred in R. rubiginosa and R. pendulina (each 80%), of prolate-spheroidal pollen in R. gallica and R. phoenicia (56.7% each), and of prolate grains in R. setigera (83.3%) and R. villosa (70%). Oblate-spheroidal and spheroidal pollen grains were found to occur most numerously in R. stellata (76.7%) and R. carolina (56.7%), whereas the highest numbers of elongated grains (from subprolate, prolate, and perprolate classes) were observed in R. blanda and R. gymocarpa (96.7% each) and R. setigera (90%). Pollen grains from the perprolate class occurred only in R. setigera (Table 5).
Exine was two-layered and well marked in LM. Ectexine and endexine are usually of the same thickness, although, sometimes, ectexine is thicker. Mean exine thickness was 1.10 (0.6–2.0) μm. Exine was thinnest in R. spinosissima (Exp–0.69 μm; Exe–0.71 μm) and thickest in R. pendulina (Exp–1.97 μm; Exe–2.0 μm) (Table 4). The average relative thickness of the exine Exp/P ratio was 0.04 (range 0.01–0.09) and of the Exe/E ratio was 0.04 (range 0.02–0.1). The above results indicated that exine was characterized by more or less identical thickness along the entire pollen grain.
In the majority of species, exine was striate, although in R. multibracteata and R. multiflora, the sculpture was striate-psilate, and blurred striae that disappeared in places were observed as well as numerous, frequently small perforations (Fig. 18). Granular-verrucate sculptures occurred only in R. stellata. Exine sculptures of this species form very numerous granules (elements <1 μm) and verrucae (elements > 1 μm) of varying diameters (Figs. 4, 19). Perforations were small and numerous (Fig. 19). Exine sculptures of striate type were very changeable (Figs. 12–18). Striae and grooves usually ran parallel to colpi and the polar axis, but frequently they also formed fingerprint-like twists. Striae were straight or forked and of varying length, width, and height. In part of the species, circular or elliptic perforations of different diameters (from 0.07 to 0.33 μm; average 0.1–0.2 μm) were found at the bottom of grooves. In the majority of the species studied the perforations were numerous with the exception of R. banksiae, R. foetida, R. persica, R. sericea, and R. spinosissima, where they were rare or did not occur at all (Figs. 12–18).
Measurements of several exine sculptural characteristics revealed that they were changeable as demonstrated by a wide range of values (Table 3). As mentioned above, perforations either did not occur or were very scarce in R. persica (subgenus Hulthemia), R. banksiae (section Banksianae), as well as in species examined from the section Pimpinellifoliae. The greatest number of perforations was found in species from the Rosa section (average 58; maximum 98). With regard to the mean number of perforations, the species studied can be divided into the following three groups (Table 3): group 1 (0–10 perforations), group 2 (25–35 perforations), and group 3 (48–58 perforations). The total area of perforations in the majority of the investigated taxa ranged, on average, from 0.4 to 0.6 μm2, with the smallest value of these features (on average 0.07 μm2) found in R. persica (subgenus Hulthemia), R. banksiae (section Banksianae), as well as species from the section Pimpinellifoliae, while the largest occurred in representatives of the Rosa and Synstylae sections (0.82 and 0.70 μm2, respectively). Numbers of striae ranged from 7 to 19 and, generally speaking, were similar in all species investigated (mean 8–13) with the exception of representatives of the Indicae section, where there were 17. The width of striae was fairly varied, ranging from 0.14 to 0.56 μm, although mean values of this feature were quite similar and fluctuated from 0.18 to 0.33 μm. Narrow striae were found, for example, in species from the Pimpinellifoliae, Rosa, and Synstylae sections (on average 0.18–0.19 μm), while the widest occurred in species from the Gallicanae and Bracteatae sections (on average 0.30–0.33 μm).
The species investigated were classified by Ueda and Tomita (1989) into five types and six subtypes of pollen exine sculpture isolated for the genus Rosa. In our study, type IV was not identified. R. banksiae, R. foetida, R. persica, and R. spinosissima belonged to type IA, R. sericea to type IB, R. acicularis, R. arkansana, R. blanda, R. canina, R. gallica, R. gymnocarpa, R. jundzillii, R. moyesii, R. nitida, R. pendulina, R. roxburghii, R. rubiginosa, R. rugosa, and R. sertata to type IIA, R. moyesii and R. pendulina to type IIB, R. agrestis, R. chinensis, R. dumalis, R. laevigata, R. phoenicia, R. setigera, R. villosa, and R. virginiana to type IIIA, R. agrestis, R. bracteata, R. canina, R. carolina, and R. dumalis to type IIIB, R. multibracteata and R. multiflora to type V, and R. stellata to type VI. Five of the 32 studied species had two types of exine sculpture (R. agrestis, R. canina, R. dumalis, R. moyesii, and R. pendulina). According to our investigation, the greatest number of species (14) belong to the IIA sculpture type, which is characterized by fairly distinct striae, narrow grooves, and frequently by prominent, numerous perforations. Type IIIA is also represented by a fairly large number of species with higher (i.e., more conspicuous than in type IIA), fairly narrow striae in comparison with type IIA. The smallest numbers of species, just one each, belong to sculpture types IB and VI.
Pollen grains usually possess three, very rarely, four apertures—colpori. Colpi were arranged meridionally, regularly, more or less evenly spaced, and were long with mean length of 25.88 (14–40) μm (Table 4; Figs. 1–5). On average, the length of colpi constituted 83.7% of the polar axis length. Generally speaking, the shortest colpi were found in R. banksiae (17.1 μm) and R. stellata (19.2 μm), while the longest were found in R. multibracteata (31.9 μm) and R. moyesii (33.2 μm). Colpi were fusiform in outline. Their width was variable and usually greatest in the equatorial region. Sculpturing of ectocolpus membranes approached regulate (Fig. 5). Frequently, the colpus membrane was partially or completely covered by an operculum. In many of the species studied (e.g., R. acicularis, R. agrestis, R. banksiae, R. canina, R. carolina, R. chinensis, R. gymnocarpa, R. multiflora, R. phoenicia, R. roxburghii, R. rubiginosa, R. sericea, and R. villosa), the colpus was crossed at the equator by a bridge dividing it into two parts, formed by two bulges of ectexine that meet in the middle (Fig. 6). The bulges were of the same or unequal length. Ectoapertures were mostly constricted at the equator by arching sexinal, narrow pore flaps. Colpus margins frequently had small undulations. Costae colpi were present (Fig. 5).
The operculum occurred in many of the species examined. There were species in which no opercula were found (e.g., R. arvensis) as well as those that possessed pollen with or without this structure (e.g., R. canina and R. rubiginosa). The operculum was usually situated in the central part of the ectocolpus; sometimes it was placed asymmetrically and partially covering the ectocolpus (Figs. 7–11). As a rule, it was an elongated structure that bulged sometimes more and, at other times, less. The operculum sculpture was typically psilate, rarely striate. Its surface was usually corrugated. Large convex operculum occurred, for example, in R. agrestis, R. canina, R. chinensis, R. gallica, R. gymnocarpa, R. jundzillii, R. multiflora, R. pendulina, R. sericea, R. spinosissima, and R. villosa, whereas narrower, elongated, and more flattened ones can be found in, e.g., R. banksiae, R. bracteata, R. pendulina, R. persica, R. roxburghii, and R. stellata.
The polar area index (PAI) or apocolpium index, in other words the d/E ratio, averaged 0.19 (0.06–0.44); averages among species ranged from 0.132 to 0.303. The lowest mean values of this index were recorded in R. carolina (0.132) and R. stellata (0.145), while the highest occurred in R. banksiae (0.303) and R. multibracteata (0.285) (Table 4).
Endoapertures were usually located in the middle of colpi, less frequently asymmetrically, usually singly (Fig. 5), very rarely in pairs. They were circular or elliptic in outline with irregular margins.
Interspecific variability of pollen grains
Statistically significant differences were found among the species examined for 10 of the pollen grain features analyzed (P < 0.0001; Table 2; features 1–10). The statistical analyses are summarized in Table 4.
Mean coefficients of variation (CV, calculated taking into account all the rose species examined) for the pollen features analyzed were: P—7.00%, E—9.61%, P/E—9.98%, Exp/P—32.20%, Exe—30.19%, ExE/E—31.35%, Le—9.26%, d—29.89%, d/E—29.53%. Exp, Exe, and d features and Exp/P, Exe/E, and d/E ratios associated with them were more variable than the remaining ones.
On the other hand, analyses of mean coefficients of variation of pollen features for individual rose species showed that pollen grains of R. pendulina (CV = 8.93%,) and R. canina (CV = 13.55%) were characterized by the lowest variability, whereas the highest variability was found in R. foetida (CV = 28.82%) and R. setigera (CV = 28.88%). Taking into consideration mean coefficients of variation, the rose species analyzed can be arranged as follows (from least to most variable): R. pendulina (CV = 8.93%), R. canina (CV = 13.55%), R. agrestis (CV = 14.16%), R. rubiginosa (CV = 15.78%), R. dumalis (CV = 16.35%), R. jundzillii (CV = 17.28%), R. sericea (CV = 19.59%), R. rugosa (CV = 19.81%), R. banksiae (CV = 20.11%), R. virginiana (CV = 20.42%), R. acicularis (CV = 21.00%), R. multiflora (CV = 21.06%), R. sertata (CV = 21.23%), R. gymnocarpa (CV = 21.46%), R. gallica (CV = 21.66%), R. phoenicia (CV = 21.73%), R. moyesii (CV = 22.22%), R. spinosissima (CV = 23.03%), R. nitida (CV = 23.31%) (CV = 21.23%), R. bracteata (CV = 23.84%), R. multibracteata (CV = 23.86%), R. roxburghii (CV = 24.26%), R. chinensis (CV = 25.07%), R. blanda (CV = 25.13%), R. stellata (CV = 25.91%), R. persica (CV = 26.59%), R. arkansana (CV = 26.86%), R. laevigata (CV = 27.89%), R. villosa (CV = 28.03%), R. carolina (CV = 28.10%), R. foetida (CV = 28.82%), and R. setigera (CV = 28.88%).
A dendrogram using Ward’s method of agglomeration (Fig. 20) was used to divide the rose species examined into three large groups, each of which is further subdivided into two or three subgroups. The first group comprises R. agrestis, R. rubiginosa (subgroup 1A), R. canina, R. dumalis, R. jundzillii, R. pendulina (subgroup 1B). The second one includes R. bracteata, R. foetida, R. persica, R. moyesii, R. setigera (subgroup 2A), R. carolina, R. virginiana, R. multiflora, R. gallica, R. rugosa, R. acicularis, R. arkansana, R. gymnocarpa, R. sertata, R. sericea, R. spinosissima (subgroup 2B), R. chinensis, R. phoenicia, R. roxburghii, R. multibracteata (subgroup 2C). The third group comprises R. banksiae (subgroup 3A), R. villosa, R. blanda, R. nitida, R. laevigata (subgroup 3B), R. stellata (subgroup 3C).
Pollen key
-
1 Exine sculpture granular-verrucate..………………….………………………………….…R. stellata
-
1* Exine sculpture different……………………….………………………………………………2
-
2 Exine sculpture striate-psilate………………………………………………R. multibracteata, R. multiflora
-
2* Exine sculpture striate ………….. …………..…………………………………………………3
-
3 Pollen grains small; average length of polar axis (P) 21.3 μm.…………….………. R. banksiae
-
3* Pollen grains medium; average length of polar axis (P) 26.5-37.8 μm…….……………………..4
-
4 Pollen grains strongly elongated (mean P/E ratio ≥ 1.5)………………………………………….R. setigera
-
4* Majority of pollen grains wider (on average P/E ratio < 1.5)………….……………………..….5
-
5 No perforations; if there are, they are scarce and usually small.……….…….….…………………6
-
5* Perforations present, often numerous of varying diameters……………………………………………….7
-
6 Grooves narrow (grooves width ± as in striae)………………R. foetida, R. persica, R. spinosissima
-
6* Grooves wide (grooves usually ≥ 2 wider than in striae)………………………………………R. sericea
-
7 Perforations often with large diameters, striae distinct………………………………………………………..8
-
7* Perforations, usually of small, varying diameters, striae convex, more conspicuous than in the previous sculpture type……………………………………………………………………………………………………..9
-
8 Grooves narrow………………………………………………………………………………………….R. acicularis, R. arkansana, R. blanda, R. canina, R. gallica, R. gymnocarpa, R. jundzillii, R. moyesii, R. nitida, R. pendulina, R. roxburghii, R. rubiginosa, R. rugosa, R. sertata
-
8* Grooves wide……………………………………………………………………………..R. moyesii, R. pendulina
-
9 Grooves narrow………………………….R. agrestis, R. bracteata, R. canina, R. carolina, R. dumalis
-
9* Grooves wide….….R. agrestis, R. chinensis, R. dumalis, R. phoenicia, R. villosa, R. virginiana.
Discussion
In the palynological literature, short morphological descriptions of pollen grains or, alternatively, only pollen dimensions are provided (usually length of polar axis and equatorial diameter) for the following species that we studied: R. acicularis, R. canina, R. foetida, R. gallica, R. multiflora, R. pendulina, R. rubiginosa, R. rugosa, R. setigera, R. spinosissima, and R. villosa (Teppner 1965; Reitsma 1966; Stachurska et al. 1974–1975, 1976; Kuprianowa and Alyoshina 1978; Eide 1981; Savitsky et al. 1987; Jones et al. 1995; Popek 1996; Shinwari and Khan 2004; Wrońska-Pilarek 2010). In studies by Wrońska-Pilarek and Boratyńska (2005) and Wrońska-Pilarek and Lira (2006), detailed palynological characteristics of R. gallica and R. pendulina were presented, which were similar to the results obtained in this study.
According to Shinwari and Khan (2004), the palynological features of exine thickness, shape, length of polar axis and equatorial diameter, and length of colpi have been found to be useful criteria for delimiting species of the genus Rosa. The results presented here corroborate this opinion with the reservation that these features allow identification of only some of the species studied.
A majority of palynologists maintain that exine sculpture features are diagnostic for pollen grains of Rosaceae at the genus as well as species level (Eide 1981; Hebda and Chinnappa 1990; Popek 1996; Ueda 1992; Ueda and Tomita 1989). The most important of them include the number and size of perforations as well as the interval, number, and diameter of striae (Fogle 1977; Matsuta et al. 1982; Marcucci et al. 1984; Menge 1985; Hebda et al. 1988; Hebda and Chinnappa 1990; Ueda and Okada 1994).
The research results obtained in this study do not corroborate the diagnostic significance of the number of perforations, found by Menge (1985), because groups of species from different subgenera and sections possess similar numbers of perforations (Table 3); for instance, species with the smallest number of perforations (0–10) include R. persica from the subgenus Hulthemia, R. banksiae from the subgenus Rosa and the section Banksianae, as well as representatives of the Rosa subgenus and Pimpinellifoliae section. On the other hand, species from five different sections (Bracteatae, Caninae, Gallicanae, Rosa, and Synstylae) representing the Rosa subgenus are characterized by the highest number of perforations (48–58). Hebda and Chinnappa (1990) distinguished two types of perforations in Rosaceae (striate sculpturing, macroperforate and nonstriate sculpturing, macroperforate, each with six subtypes) possibly indicating different evolutionary lines. They included roses in the first type with large perforations often extending onto tectal striae. In addition, they also drew attention to the fact that, in the case of Rosa, striae are long and parallel to the colpus. According to the above-cited study, pollen of Rosa (with Prunus, Rubus, and Spirea) belongs to the subcategory with striae separated by grooves, containing larger perforations (0.1–0.2 μm in diameter). Results reported by Wrońska-Pilarek (2010) as well as the current data corroborated this latest thesis, with the reservation that some of the species are characterized by sculpture different from striate, and that because perforation diameters differ so much in Rosa (ranging from 0.07 to 0.33 μm), it is necessary to specify that “larger” refers to mean values of perforation diameters of 0.1–0.2 μm. Hebda and Chinnappa (1994), using differences in supratectal elements and perforation form and size, classify pollen types in Rosaceae into six main categories: 1—striate and macroperforate, 2—striate and microperforate, 3—tuberculate and perforate, 4—microverrucate, 5—verrucate, and 6—perforate, without supratectal features. They included species from the Rosa genus, similarly to the study from 1990, in type 1 (striae long and parallel to colpus). Our studies demonstrated that the inclusion of the Rosa genus into one type is too general because, firstly, there are rose species with exine sculpture granular-verrucate (e.g. R. stellata) or striate-psilate (e.g., R. multibracteata and R. multiflora) and perforations are sometimes large but also small (type 2, striate and microperforate), and in some species perforations are very scarce or do not occur at all (e.g., R. foetida and R. persica). Consequently, species from the Rosa genus also belong to other types mentioned above as well as to types not mentioned by Hebda and Chinnappa (1994).
Ueda and Tomita (1989) distinguished six types and three subtypes of exine sculpture in species and other taxa from the Rosa genus. The above experiments were later continued by Ueda (1992), who classified rose species investigated in this study into all six types distinguished by him. In the current study, they were classified into five types (type IV was not distinguished). The majority of species were included in the same types as the above-mentioned author, although seven species were included in other types (R. acicularis—not IIB but IIA, R. banksiae—not IIIA but IA, R. bracteata and R. carolina—from IV to IIIB, R. gymnocarpa—from V to IIA, R. laevigata—from IIA to IIIA, and R. virginiana—from IV to IIIA) and four were classified both to the type proposed by Ueda (1992) and to yet another type (R. agrestis—IIIB and IIIA, R. canina—IIA and IIIB, R. moyesii—IIA and IIB, and R. pendulina—IIA and IIB). Ueda (1992) did not quote sculpture types mentioned in this publication for R. dumalis and R. phoenicia. Differences in the classification of individual species can contribute to considerable inter- and intraspecific variability of exine sculpture features. Popek (1996) distinguished two types of sculpture in roses: reticulate and striate, the latter with 11 subtypes (6 species representing 6 subtypes were analyzed in the current article: R. banksiae, R. bracteata, R. gallica, R. gymnocarpa, R. laevigata, and R. sericea). The division proposed by Popek (1996) partly corresponds to Ueda’s (1992) classification but it is descriptive and not based on measurements of exine sculpture features and, therefore, it is difficult to compare these two classifications. That is why results obtained in our studies were compared with Ueda’s (1992) classification, because in the cited study and in our study we do not distinguish reticulate sculpture for species from the Hesperrhodos subgenus (R. minutifolia and R. stellata). Ueda and Tomita (1989) listed reticulate sculpture for these species, but in our opinion, the sculpture is granulate-verrucate because it is made up not only of verrucae—larger elements with diameters smaller than 1 μm, but also numerous granules measuring less than 1 μm (Fig. 19).
Recapitulating, the results of current investigations only partially corroborate the diagnostic significance of exine sculpture features, not only at the level of subgenus or section, but also at the species level. The exception is R. stellata from the subgenus Hesperrhodos, which, according to Zieliński (1985), phylogenetically represents the oldest group of the Rosa genus which possesses granulate-verrucate, and not striate sculpture as nearly all the remaining species. Also, R. multibracteata and R. multiflora have specific sculpturing which was classified as the striate-psilate type. R. persica from the Hulthemia subgenus has exine sculpture of the IA type, and R. roxburghii from the Platyrhodon subgenus has type IIA. Both these types also occur in other species from the Eurosa subgenus (IA in two species from section Pimpinellifoliae, and IIA in the majority of the species investigated from this subgenus). From among the species of the Eurosa subgenus studied by us, the rarest sculpture types included IB (R. sericea), IIB (R. acicularis), and V (R. multibracteata and R. multiflora); only slightly more species were found to belong to the IA and IIIB types. Such classification is only partly confirmed by Ueda (1992). This refers to the IB and IIB types which accounted for only 2 and 5 out of the 126 taxa studied by the above-mentioned author from the genus Rosa, whereas type V accounted for up to 15 species from sections Carolinae and Rosa. On the other hand, his IA type is quite numerous (14 species), whereas type IIIB is sparse (7 species). Investigations revealed that exine sculpture features are subject to significant variability which reduces their diagnostic value. That is why exine sculpture should be treated as an auxiliary feature, because usually it fails to distinguish single species. On the other hand, it is useful when groups of species of similar exine sculpture are to be identified.
Popek (1996) distinguished six operculum types (R. banksiae, R. canina, R. foetida, R. kostrakiewiczii, R. sericea, and R. spinosissima type) in roses. In our study, despite the observed operculum structure variability, it was not used as a basis for identification of groups of species, because this structure is very variable and it is quite easy to make a mistake in its diagnosis. The operculum may be mistaken for structures of other origin, e.g., with bulges of the intine at the beginning of pollen tube germination. It usually appears in mature pollen grains, so it may not be noticed when analyzing pollen at other developmental stages. There are sparse and frequently contradictory data about which rose species have and which do not have this structure. Eide (1981) maintains that R. canina, R. spinosissima, and R. rubiginosa have pollen with or without an operculum and adds that, in R. canina, operculate pollen seems to be less frequent than pollen without the operculum. Reitsma (1966) and Hebda and Chinnappa (1994) write that both pollen with the operculum (e.g., R. canina and R. gallica) and without it (R. rubiginosa, R. rugosa, and R. arvensis) are found. In addition, it turns out that, in the same species (e.g., R. pendulina), both large and bulged as well as narrow and flat opercula can occur.
The distribution of the species investigated on the dendrogram corroborates only slightly the division of the Rosa genus currently adopted in taxonomy into subgenera and sections (Rehder 1940). R. persica, from the subgenus Hulthemia, is found in group 2, the group with the most species (subgroup 2A), together with its closest relatives R. bracteata and R. foetida, which derive from two sections of the Eurosa subgenus (Fig. 20). R. roxburghii from the Platyrhodon subgenus with features closest to R. chinensis and R. phoenicia pollen derives from two different sections of the Eurosa subgenus, but belongs to the same group 2 in our dendrogram. R. stellata from the Hesperrhodos subgenus is found in group 3, where it is isolated in its own subgroup, and the features of its pollen grains are closest to R. laevigata and R. nitida, as well as to R. blanda and R. villosa, i.e., species representing four different sections from the Eurosa subgenus. On the other hand, species from 10 sections of the subgenus Eurosa were placed in all three groups. An interesting result was recorded for the species studied from the Caninae section (R. agrestis, R. canina, R. dumalis, R. jundzillii, and R. rubiginosa) which, with the exception of R. villosa (subgroup 3B), were found in the same group of species. It is worth emphasizing that the above-mentioned species, contrary to current opinions, are characterized by the smallest mean values of the coefficient of variability. The only other species found in this group was R. pendulina from the Rosa section (syn. Cinnamomeae). Its presence in this group would confirm Zieliński’s thesis (1985, 1987) about the lack of a morphological boundary between the Caninae section and groups that contributed to its establishment, in particular the section Rosa. However, the membership of the remaining species from the Rosa section (with the exception of R. blanda, group 3B) in the second group makes it impossible to draw unequivocal conclusions.
Gustafsson (1944), Zieliński (1985), and Henker (2000) investigated consanguinity relationships between the species studied from the section Caninae DC. em. Christ. According to Zieliński (1985), the “initial” species for this section is R. canina from which six developmental lines depart and form R. judzillii, then R. micrantha and R. rubiginosa, R. agrestis and R. inodora, R. tomentosa, R. sherardii, and R. villosa, as well as two single species R. dumalis and R. glauca. According to Wrońska-Pilarek (2010), pollen grain morphological structure reflects these dependencies only slightly. A similar result was obtained in the current study (Fig. 20). On the one hand, species closely related to one another (e.g., R. agrestis and R. rubiginosa) were allocated to the same group, while R. judzillii was separated, although its pollen is most similar to the pollen of R. pendulina from the Rosa section. On the other hand, R. villosa is situated in a separate group 3 (subgroup 3B) together with R. blanda from the Rosa section, which constitutes a separate developmental group, R. dumalis with features closest to R. canina. These ambiguous results can be attributed to the fact that the Caninae section is by nature a swarm of R. canina hybrids with species from the Rosa, Synstylae, and Gallicanae sections, with R. canina as a link connecting taxa among the sections (Zieliński 1985, 1987).
According to Popek (1996), R. sericea (lack of perforations, operculum with strips) and R. foetida (granules of rice-shaped colpus membrane, triangular operculum) occupy an isolated position due to specific features of their exine sculpture and operculum. In his opinion, the above-mentioned features of R. sericea and R. foetida allow isolation from the Pimpinellifoliae section and their inclusion in separate sections, i.e., Sericeae and Luteae. An additional argument in support of such a measure is the fact that pollen grains of these species differ with respect to their structure from pollen grains typical for the R. pimpinellifoliae (syn. R. spinosissima) section, having other operculum and grooves without or with sparse perforations. Results of investigations presented in this paper do not corroborate this concept, firstly because R. foetida, R. spinosissima (type IA), and R. sericea (type IB) possess very similar sculpture, and secondly because opercula described by Popek (1996) as R. sericea type, i.e. “on the side, surrounded by strips” (Fig. 7), and R. foetida type probably occurred in specific pollen grains examined by that author; our investigations in both these species as well as in R. spinosissima revealed opercula similar to in, among others, R. canina or R. multiflora (Fig. 9).
According to Popek (1996), R. gymnocarpa, due to its exine structure and other features, should also be included in a separate Gymnocarpae section which, among others, Lewis (1957) included, together with the Carolinae section, in the section Cinnamomeae (syn. Rosa). This was the classification adopted here, but the palynological studies performed failed to confirm the hypothesis of a separate identity of this species or its similarity to species from the Carolinae and Rosa sections.
References
Bai JR, Zhang QX, Luo L, Pan HT, Yu C (2011) Pollen morphology of some Chinese traditional roses. Bull Bot Res 31(10):15–23
Eide F (1981) Key for Northwest European Rosaceae pollen. Grana 20:101–118
Erdtman G (1952) Pollen morphology and plant taxonomy. Angiosperms. An introduction to palynology 1. Almquist and Wiksell, Stockholm
Faegri K, Iversen J (1989) Textbook of pollen analysis. Wiley, Chichester
Fogle HW (1977) Identification of clones within four tree species by pollen exine patterns. J Am Soc Hort Sci 102:552–560
Gonzalez Romano ML, Candau PA (1989) Contribution to palynological studies in the Rosaceae. Acta Bot Malacit 14:105–116
Gustafsson A (1944) The Constitution of the Rosa canina complex. Hereditas 30:411–412
Hebda RJ, Chinnappa CC (1990) Studies on pollen morphology of Rosaceae in Canada. Rev Paleobot Palynol 64:103–108
Hebda RJ, Chinnappa CC (1994) Studies on pollen morphology of Rosaceae. Acta Bot Gall 141:183–193
Hebda RJ, Chinnappa CC, Smith BM (1988) Pollen morphology of the Rosaceae of Western Canada I. Agrimonia to Crataegus. Grana 27:93–113
Henker H (2000) Rosa. In: Hegi G (ed) Illustrierte Flora von Mittel-Europa. Band IV, Teil 2C, Lieferung A, Bg 1-7 Parey Buchverlag, Berlin
Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S (2009) Pollen terminology. An illustrated handbook. Springer, Vienna
Hutchinson J (1964) The genera of flowering plants. Oxford University Press, Oxford
Jones GD, Bryant VM Jr, Lieux MH, Jones SD, Lingren PD (1995) Pollen of the Southeastern United States. AASP Contributions. 30
Kalkman C (2004) Rosaceae. In: Kubitzki K (ed) The families and genera of vascular plants. VI Flowering plants—dicotyledons. Springer, Berlin, Heidelberg, pp 386–443
Kuprianowa LA, Alyoshina LA (1978) Pollen dicotyledonarum florae partis Europeae URSS Lamiaceae–Zygophyllaceae. Komorovii Institutum Botanicum, Academia Scientiarum USSR
Lewis WH (1957) Revision of the genus Rosa in eastern North America. A review. Am Rose Ann 42:116–126
Marcucci MC, Sansavini S, Ciampolini F, Cresti M (1984) Distinguish apple clones and cultivars by surface morphology and pollen physiology. J Am Soc Hort Sci 109:10–19
Matsuta N, Omura M, Akihama T (1982) Difference in micromorphological pattern on pollen surface of Japanese Pear cultivars. Jpn J Breed 32:123–128
Menge U (1985) Identifizierung von Rosensorten anhand von Pollenoberflächenmuster. Gartenbauwissenschaft 501:1–9
Moore PD, Webb JA, Collinson ME (1991) Pollen analysis. Blackwell Scientific, London
Nicolson DH (1992) Seventy-two proposals for the conservation of types of selected Linnaean generic names, the report of Subcommittee 3C on the lectotypification of Linnaean generic names. Taxon 41:552–583
Nilsson O (1997) Rosa. In: Davis PH (ed) Flora of Turkey and the East Aegean Islands, vol 4. Edinburgh University Press, Edinburgh, pp 106–128
Popek R (1996) Biosystematyczne studia nad rodzajem Rosa L w Polsce i krajach ościennych. WN WSP, Kraków
Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A (2007) Glossary of pollen and spore terminology. Rev Palaeobot Palynol 1431(2):1–81
Rehder A (1940) Manual of cultivated trees and shrubs, 2nd edn. Collier Macmillan, New York
Rehder A (1949) Manual of cultivated trees and shrubs hardy in the cooler temperate regions of the Northern Hemisphere. Arnold Arboretum, Jamaica Plain, MA, USA
Reitsma TJ (1966) Pollen morphology of some European Rosaceae. Acta Bot Neerl 15:290–379
Rowley GD (1976) Typification of the genus Rosa L. Taxon 25(1):181
SAS Institute Inc. Cary, NC, USA.; http://www.jmp.com/
Savitsky VD, Dubovik OM, Fedoronchuk MM (1987) Pollen morphological diversity of the genus Rosa L. in Ukrainien flora. Ukr Bot Zh 431:36–41
Shinwari M, Khan MA (2004) Pollen morphology of wild roses from Pakistan. Hamdard Medicus 474:5–13
Stachurska A, Sadowska A, Kuszell T (1974–1975) The palynological card index of Polish plants. Opol Tow Przyj Nauk 14–15, Wrocław: Tables: 214–223
Stachurska A, Sadowska A, Kuszell T (1976) The palynological card index of Polish plants. Opol Tow Przyj Nauk 16, Wrocław: Tables: 224–233
Teppner H (1965) Zur Kenntnis der Gattung Waldsteinia L. Phyton 34:224–238
Ueda Y (1992) Pollen surface morphology in the genus Rosa related genera. Jpn J Palynol 38(2):94–105
Ueda Y, Okada Y (1994) Discrimination of rose cultivar groups by pollen surface structure. J Hort Sci 694:601–607
Ueda Y, Tomita H (1989) Morphometric analysis of pollen patterns in roses. J Jpn Soc Hort Sci 581:211–220
Wodehouse RP (1935) Pollen grains. Their structure, identification and significance in science and medicine. McGraw-Hill, New York
Wrońska-Pilarek D (1998) Pollen morphology of the Polish species of the genus Ribes L. Acta Soc Bot Polon 67(3–4):275–285
Wrońska-Pilarek D (2010) Pollen morphology of Polish native species of the Rosa genus (Rosaceae) and its relation to systematic. Acta Soc Bot Polon 79 (in press)
Wrońska-Pilarek D, Boratyńska K (2005) Pollen morphology of Rosa gallica L. Rosaceae L. from southern Poland. Acta Soc Bot Polon 744:297–304
Wrońska-Pilarek D, Jagodziński AM (2009) Pollen morphological variability of Polish native species of Rosa L. (Rosaceae). Dendrobiology 62:71–82
Wrońska-Pilarek D, Lira J (2006) Pollen morphology of Polish species of the genus Rosa L. I—Rosa pendulina L. Dendrobiology 55:65–73
Zhou LH, Wie ZX, Wu ZY (1999) Pollen morphology of Rosoideae (Rosaceae) of China. Acta Bot Yunnan 21(4):455–460
Zieliński J (1985) Studia nad rodzajem Rosa L.—Systematyka sekcji Caninae DC em Christ. Arbor Kórn 30:3–109
Zieliński J (1987) Rodzaj Rosa L. In: Jasiewicz A (ed) Flora Polski. Rośliny naczyniowe. PWN, Warszawa-Kraków, pp 7–49
Acknowledgments
We would like to thank the following people for help in research work: the scientists of the Institute of Botany of Vienna University, especially Prof. Michael Hesse and Dr. Heidemarie Halbritter, Dr. Walter Till (Herbarium University of Vienna), and Prof. Ryszard Popek (Pedagogical University of Cracow). We kindly thank Dr. Lee E. Frelich (University of Minnesota, USA) for linguistic support. We would like to thank the reviewers for their detailed and valuable comments on the manuscript. The work was supported by the State Committee for Scientific Research (KBN) grant no. 2 P04C 084 30.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wrońska-Pilarek, D., Jagodziński, A.M. Systematic importance of pollen morphological features of selected species from the genus Rosa (Rosaceae). Plant Syst Evol 295, 55–72 (2011). https://doi.org/10.1007/s00606-011-0462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0462-y