Abstract
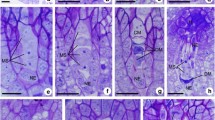
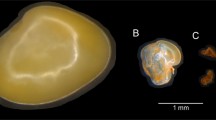
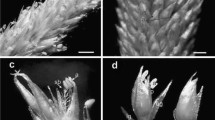
Handroanthus chrysotrichus shows pollination-dependent self-fertility, polyploidy, and adventitious polyembryony, and it is closely related to H. ochraceus, for which apparently conflicting reports of self-incompatibility and apomixis have been published. The present study aims to investigate the polyembryony in these species by means of histological analysis of ovule/seed development in unpollinated, selfed, and crossed pistils/fruits (in H. chrysotrichus only) as well as seed germination experiments. Experimental pollinations were carried out to evaluate breeding systems in the studied populations, and the results indicated self-fertility in both species. Adventitious embryo precursor cells (AEPs) were formed in the ovules of unpollinated, selfed, and crossed pistils. However, unfertilized ovules never develop into seeds, and fertilization/endosperm initiation clearly stimulates the formation of AEPs in pollinated pistils. The inability of AEP-bearing unfertilized ovules to initiate endospermogenesis clearly shows that fertilization is needed for adventitious embryo development. Consequently, formation of AEPs is required but is not sufficient for apomictic reproduction in H. chrysotrichus. Analysis of the positions of multiple embryos in the endosperm indicated that fertilized ovules are able to develop into seeds even in the absence of a zygotic embryo. The development of AEPs in ovules of H. chrysotrichus foregoes the stage in which activation of selfed pistil rejection takes place in H. impetiginosus, a species with late-acting self-incompatibility. Our study supports the hypothesis that the self-fertility in H. chrysotrichus (and perhaps also in H. ochraceus) resulted from the emergence of pseudogamous apomixis, favored by the physiological peculiarities of the late-acting self-incompatibility and possibly related to polyploidy.






Similar content being viewed by others
References
Amaral MEC (1992) Ecologia floral de dez espécies da tribo Bignoniae (Bignoniaceae) em uma floresta semidecídua no município de Campinas, São Paulo. Dissertation, Universidade Estadual de Campinas
Asker SE, Jerling L (1992) Apomixis in plants. CRC, Boca Raton
Bawa KS (1974) Breeding systems of tree species of lowland tropical community. Evolution 28:85–92
Bawa KS, Webb CJ (1984) Flower, fruit and seed abortion in tropical forest trees: Implications for the evolution of paternal and maternal reproductive patterns. Am J Bot 71:736–751
Bertin RI, Sullivan M (1988) Pollen inference and cryptic self-fertility in Campsis radicans. Am J Bot 75:1140–1147
Bittencourt NS Jr, Semir J (2004) Pollination biology and breeding system of Zeyheria montana (Bignoniaceae). Plant Syst Evol 247:241–254. doi:10.1007/s00606-004-0142-2
Bittencourt NS Jr, Semir J (2005) Late-acting self-incompatibility and other breeding systems in Tabebuia (Bignoniaceae). Int J Plant Sci 166:493–506
Bittencourt NS Jr, Semir J (2006) Floral biology and late-acting self-incompatibility in Jacaranda racemosa (Bignoniaceae). Aust J Bot 54:315–324
Bittencourt NS Jr, Gibbs PE, Semir J (2003) Histological study of post-pollination events in Spathodea campanulata Beauv. (Bignoniaceae), a species with late-acting self-incompatibility. Ann Bot 91:827–834
Bullock SH (1985) Breeding systems in flora of tropical deciduous forest in Mexico. Biotropica 17:287–301
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyploidy. Biol J Linn Soc 61:51–94
Costa ME, Sampaio DS, Paoli AAS, Leite SCAL (2004) Poliembrionia e aspectos da embriogênese em Tabebuia ochracea (Cham.) Standley (Bignoniaceae). Rev Bras Bot 27:395–406
de Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants, 2nd edn. Springer, Berlin
Dutra JCS, Machado VLL (2001) Entomofauna visitante de Stenolobium stans (Juss.) Seem (Bignoniaceae), durante seu período de floração. Neotropical Entomol 30:43–53
Garcia R, Asins MJ, Foener J, Carbonell EA (1999) Genetic analysis in Citrus and Poncirus by genetic markers. Theor Appl Genet 99:511–518
Gentry AH (1974) Coevolutionary patterns in Central American Bibnoniaceae. Ann Missouri Bot Gard 61:728–759
Gentry AH (1992) Bignoniaceae—Part II (Tribe Tecomeae). Flora Neotropica: Monograph 25 (II). Organization for Flora Neotropica, New York
Gibbs PE, Bianchi M (1993) Post-pollination events in species of Chorisia (Bombacaceae) and Tabebuia (Bignoniaceae) with Late-acting self-incompatibility. Bot Acta 106:64–71
Gibbs PE, Bianchi M (1999) Does late-acting self-incompatibility (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa. Ann Bot 84:449–457
Gobatto-Rodrigues AA, Stort MN (1992) Biologia floral e reprodução de Pyrostegia venusta (Ker-Gawl) Miers (Bignoniaceae). Rev Bras Bot 15:37–41
Goldblatt P, Gentry AH (1979) Cytology of Bignoniaceae. Bot Not 132:475–482
Grose SO, Olmstead RG (2007) Taxonomic revisions in the polyphyletic genus Tabebuia s. l. (Bignoniaceae). Syst Bot 32:660–670
Guimarães E, Stasi LC, Maimoni-Rodella RCS (2008) Pollination biology of Jacaranda oxyphylla with an emphasis on staminode function. Ann Bot 102:699–711
Hörandl E (2009) The evolution of self-fertility in apomictic plants. Sex Plant Reprod (in press). doi:101007/s00497-0090122-3
James EA, Knox RB (1993) Reproductive Biology of the Australian species of the genus Pandorea (Bignoniaceae). Aust J Bot 41:611–626
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Johansen DA (1950) Plant embryology. Chronica Botanica, Waltham
Johri BM, Ambegaokar KB, Srivastava PS (1992) Comparative embryology of angiosperms, vol 2. Springer, Berlin
Klekowski EJ Jr (1988) Mutation, developmental selection, and plant evolution. Columbia University Press, New York
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574. doi:10.1146/annurev.arplant.54.110901.160842
Lakshmanan KK, Ambegaokar KB (1984) Polyembryony. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 445–474
Lorenzi H (2002) Árvores brasileiras, vol. 1, 4th edn. Plantarum, Nova Odessa
Maurizon J (1935) Etwas über die Embryologie der Bignoniaceen. Bot Not 1935:60–77
Mendes-da-Glória FJ, Mourão Filho FAA, Appezato-da-Glória B (2001) Morfologia de embriões nucelares de laranja (Citrus sinensis L.) Osbeck. Acta Bot Bras 15:17–25
Mendes-Rodrigues C, Carmo-Oliveira R, Talavera S, Arista M, Ortiz PL, Oliveira PE (2005) Polyembryony and apomixis in Eriotheca pubescens (Malvaceae–Bombacoideae). Plant Biol 7:533–540. doi:10.1055/s-2005-865852
Milet-Pinheiro P, Schlindwein C (2009) Pollination in Jacaranda rugosa (Bignoniaceae): euglossine pollinators, nectar robbers and low fruit set. Plant Biol 11:131–141. doi:10.111/j.1438-8677.2008.00118.x
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by Toluidine Blue O. Protoplasma 59:367–373
Oliveira PE, Gibbs PE, Barbosa AA, Talavera S (1992) Contrasting breeding systems in two Eriotheca (Bombacaceae) species of Brazilian cerrados. Plant Syst Evol 179:207–219
Olmstead RG, Zjhra ML, Lohmann LG, Grose SO, Eckert AJ (2009) A molecular phylogeny and classification of Bignoniaceae. Am J Bot 96:1731–1743. doi:10.3732/ajb.0900004
Petersen C, Brown JH, Kodric-Brown A (1982) An experimental study of floral display and fruit set in Chilopsis linearis (Bignoniaceae). Oecologia 55:7–11
Piazzano M (1998) Números cromosómicos em Bignoniaceae de Argentina. Kurtziana 26:179–189
Polatto LP, Alves VV Jr (2009) Sistema reprodutivo de Sparatosperma leucanthum (Vell.) K. Schum. (Bignoniaceae). Rev Árvore 33:289–296
Richards AJ (1997) Plant breeding systems. Chapman and Hall, London
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Salomão AN, Allem AC (2001) Polyembryony in angiospermous trees of the Brazilian cerrado and caatinga vegetation. Acta Bot Bras 15:369–378
Sampaio DS, Costa ME, Paoli AAS (2007) Ontogenia da semente de Tabebuia ochracea (Cham.) Standl. (Bignoniaceae). Rev Bras Bot 30:289–302
Seavey SR, Bawa KS (1986) Late-acting self-incompatibility in angiosperms. Bot Rev 52:195–219
Shivaramiah G (1998) Endosperm development in Bignoniaceae. Phytomorphology 48:45–50
Singh J, Chauhan SVS (1996) Morphological changes in the stigma of seasonaly transient sterile Tecoma stans L. Phytomorphology 46:1–7
Souza LA, Iwazaki MC, Moscheta IS (2005) Morphology of the pericarp and seed of Tabebuia chrysotricha (Mart. ex DC.) Standl. (Bignoniaceae). Braz Arch Biol Technol 48:407–418
Srithongchauay T, Bumrungsri S, Sripao-raya E (2008) The pollination ecology of the late-successional tree, Oroxylum indicum (Bignoniaceae) in Thailand. J Trop Ecol 24:477–484. doi:10.1017/S026646740800521X
Stephenson AG, Thomas WW (1977) Diurnal and nocturnal pollination of Catalpa speciosa (Bignoniaceae). Syst Bot 2:191–198
Vieira MF, Meira RMSA, Queiroz LP, Meira Neto JAA (1992) Polinização e reprodução de Jacaranda caroba (Vell.) DC. (Bignoniaceae) em área de cerrado do sudeste brasileiro. An 8th Congr SBPC 13–19
Vikas, Gautam M, Tandon R, Ram HYM (2009) Pollination ecology and breeding system of Oroxylum indicum (Bignoniaceae) in the foothills of the Western Himalaya. J Trop Ecol 25:93–96. doi:10.1017/S0266467408005634
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey
Acknowledgments
We are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support, and for a fellowship granted to the second author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bittencourt, N.S., Moraes, C.I.G. Self-fertility and polyembryony in South American yellow trumpet trees (Handroanthus chrysotrichus and H. ochraceus, Bignoniaceae): a histological study of postpollination events. Plant Syst Evol 288, 59–76 (2010). https://doi.org/10.1007/s00606-010-0313-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0313-2