Abstract
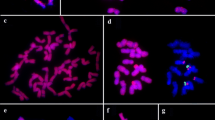
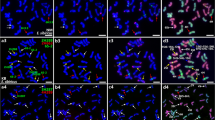
Genomic in situ hybridization (GISH) with Secale cereale cv. ‘Jingzhou rye’ DNA as a probe to chromosomes of hexaploid triticale line Fenzhi-1 revealed that not only were all chromosomes of rye strongly hybridized along the entire chromosome length, but there were also stronger signals in terminal or subtelomeric regions. This pattern of hybridization signals is referred to as GISH banding. After GISH banding, sequential fluorescene in situ hybridizaion (FISH) with tandem repeated sequence pSc200 and pSc250 as probes showed that the chromosomal distribution of pSc200 is highly coincident with the GISH banding pattern, suggesting that GISH banding revealed chromosomal distribution of pSc200 in rye. In addition, FISH using pSc200 and pSc250 as probes to chromosomes of 11 species of the genus Secale and two artificial amphiploids (Triticum aestivum-S. strictum subsp. africanum amphiploid and Aegilops tauschii-S. silvestre amphiploid) showed that (1) the chromosomal distribution of pSc200 and pSc250 differed greatly in Secale species, and the trend towards an increase in pSc200 and pSc250 binding sites from wild species to cultivated rye suggested that pSc200 and pSc250 sequences gradually accumulated during Secale evolution; (2) the chromosomal distribution of pSc200 and pSc250 presented polymorphism on homologous chromosomes, suggesting that the same species has two heterogeneous homologous chromosomes; (3) the intensity and number of hybridization signals varied differently on chromosomes between pSc200 and pSc250, suggesting that each repetitive family evolved independently.



Similar content being viewed by others
References
Alkhimova AG, Heslop-Harrison JS, Shchapova AI, Vershinin AV (1999) Rye chromosome variability in wheat-rye addition and substitution lines. Chromosome Res 7:205–212
Alkhimova OG, Mazurok NA, Potapova TA, Zakian SM, Heslop-Harrison JS, Vershinin AV (2004) Diverse patterns of the tandem repeats organization in rye chromosomes. Chromosoma 113:42–52
Appels R, Dennis ES, Smith DR, Peacock WJ (1981) Two repeated DNA sequences from the heterochromatic regions of rye, Secale cereale, chromosomes. Chromosoma 84:265–277
Bedbrook JR, Jones J, O’Dell M, Thompson R, Flavell R (1980) A molecular description of telomeric heterochromatin in Secale species. Cell 19:545–560
Bennetzen JL (1996) The contribution of retroelements to plant genome organization, function and evolution. Trends Microbiol 4:347–353
Bennetzen JL, Freeling M (1997) The unified grass genome: synergy in synteny. Genome Res 7:301–306
Charlesworth B, Sniegowski P, Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371:215–220
Chen Q, Conner RL, Li HJ, Sun SC, Ahmad F, Laroche A, Graf RJ (2003) Molecular cytogenetic discrimination and reaction to wheat streak mosaic virus and the wheat curl mite in Zhong series of wheat-Thinopyrum intermedium partial amphiploids. Genome 46:135–145
Cuadrado A, Jouve N (1995) Fluorescent in situ hybridization and C-banding analyses of highly repetitive DNA sequences in the heterochromatin of rye (Secale montanum Guss.) and wheat incorporating S. montanum chromosome segments. Genome 38:795–802
Cuadrado A, Jouve N (2002) Evolutionary trends of different repetitive DNA sequences during speciation in the genus Secale. J Hered 93:339–345
Doolittle WF, Sapienza C (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284:601–603
Flavell R (1980) The molecular characterization and organization of plant chromosomal DNA sequences. Annu Rev Plant Physiol 31:569–596
Flavell RB, Gale M, O’Dell M, Murphy G, Moore G, Lucas H (1993) Molecular organization of genes and repeats in the large cereal genomes and implications for the isolation of genes by chromosome walking. Chromosomes Today 11:199–214
Frederiksen S, Petersen G (1997) Morphometrical analyses of Secale (Triticeae, Poaceae). Nord J Bot 17:185–198
Frederiksen S, Petersen G (1998) A taxonomic revision of Secale (Triticeae, Poaceae). Nord J Bot 18:399–420
Frello S, Heslop-Harrison JS (2000) Chromosomal variation in Crocus vernus Hill (Iridaceae) investigated by in situ hybridization of rDNA and a tandemly repeated sequence. Ann Bot 86:317–322
Heslop-Harrison JS (2000) Comparative genome organization in plants: from sequence and markers to chromatin and chromosomes. Plant Cell 12:617–636
Jiang HR, Kong XX (1991) A new species of Triticale (in Chinese with English abstract). J Sichuan Agric Univ 9:334–337
Jiang HR, Dai DQ, Sun D-F (1992) Creation of special germplasm resources in Triticum (in Chinese with English abstract). J Sichuan Agric Univ 10:255–259
Jiang J, Hulbert HS, Gill BS, Ward DC (1996) Interphase fluorescence in situ hybridization: a physical mapping strategy for plant species with large complex genomes. Mol Gen Genet 252:497–502
Jones JDG, Flavell R (1982) The mapping of highly-repeated DNA families and their relationship to C-bands in chromosomes of Secale cereale. Chromosoma 86:595–612
Kidwell KK, Osborn TC (1992) Simple plant DNA isolation procedures. In: Beckmann JS, Osborn TC (eds) Methods for genetic and physical mapping. Kluwer, Dordrecht, pp 1–13
Kotseruba V, Pistrick K, Blattner FR, Kumke K, Weiss O, Rutten T, Fuchs J, Endo T, Nasuda S, Ghukasyan A, Houben A (2010) The evolution of the hexaploid grass Zingeria kochii (Mez) Tzvel. (2n = 12) was accompanied by complex hybridization and uniparental loss of ribosomal DNA. Mol Phylogenet Evol. doi:10.1016/j.ympev.2010.-01.003
Kuipers AGJ, van Os DPM, de Jong JH, Ramanna MS (1997) Molecular cytogenetics of Alstroemeria: identification of parental genomes in interspecific hybrids and characterization of repetitive DNA families in constitutive heterochromatin. Chromosome Res 5:31–39
Lapitan NLV (1992) Organization and evolution of higher plant nuclear genomes. Genome 35:171–181
McIntyre CL, Pereira S, Moran L, Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33:317–323
Minelli S, Ceccarelli M, Mariani M, De Pace C, Cionini PG (2005) Cytogenetics of Triticum × Dasypyrum hybrids and derived lines. Cytogenet Genome Res 109:385–392
Orgel LE, Crick FHC (1980) Selfish DNA: the ultimate parasite. Nature 284:604–607
Petersen G, Doebley JF (1993) Chloroplast DNA variation in the genus Secale (Poaceae). Plant Syst Evol 187:115–125
Pickering RA, Malyshev S, Künzel G, Johnston PA, Korzun V, Menke M, Schubert I (2000) Locating introgressions of Hordeum bulbosum chromatin with the H. vulgare genome. Theor Appl Genet 100:27–31
Schwarzacher T, Anamthawat-Jonsson K, Harrison GE, Islam AKMR, Jia JZ, King IP, Leitch AR, Miller TE, Reader SM, Rogers WJ, Shi M, Heslop-Harrison JS (1992) Genomic in situ hybridization to identify alien chromosomes and chromosome segments in wheat. Theor Appl Genet 84:778–786
Singh RJ, Röbbelen G (1977) Identification by Giemsa technique of the translocations separating cultivated rye from three wild species of Secale. Chromosoma 59:217–229
Vershinin AV, Schwarzacher T, Heslop-Harrison JS (1995) The large-scale genomic organization of repetitive DNA families at the telomeres of rye chromosomes. Plant Cell 7:1823–1833
Vershinin AV, Alkhimova EG, Heslop-Harrison JS (1996) Molecular diversification of tandemly organized DNA sequences and heterochromatic chromosome regions in some Triticeae species. Chromosome Res 4:517–525
Yang ZJ, Li GR, Jiang HR, Ren ZL (2001a) Expression of nucleolus, endosperm storage proteins and disease resistance in an amphiploid between Aegilops tauschii and Secale silvestre. Euphytica 119:317–321
Yang ZJ, Li GR, Ren ZL (2001b) Identification of Triticum durum-Secale africanum amphiploid and its crossability with common wheat. J Genet Breed 55:45–50
Zhou JP, Yang ZJ, Zhang XZ, Feng J, Li GR, Ren ZL (2007) Morphological, cytogenetic and molecular identification of a new Triticale. Cereal Res Commun 35:1385–1395
Acknowledgments
We are thankful to the National Natural Science Foundation of China for their financial support (No. 30800685 and No. 30730065) and we appreciate Dr. Frank Blattner and Dr. Bernd R. Friebe for their help in revising this paper.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, J., Yang, Z., Li, G. et al. Diversified chromosomal distribution of tandemly repeated sequences revealed evolutionary trends in Secale (Poaceae). Plant Syst Evol 287, 49–56 (2010). https://doi.org/10.1007/s00606-010-0288-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0288-z