Abstract
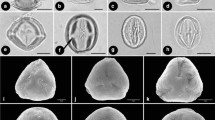
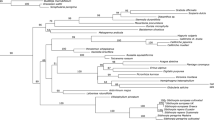
Pollen grains of tribe Sanguisorbeae (Rosaceae, Rosoideae) were examined using scanning electron microscopy to identify useful characters, test taxonomic and phylogenetic hypotheses among genera, and elucidate pollen character evolution based on a molecular phylogeny. Aperture number, aperture structure, pollen shape, and exine sculpturing were variable within Sanguisorbeae and were used to delineate six pollen types. Four types (I–IV) were observed only in subtribe Sanguisorbinae whereas two types (V–VI) were found only in subtribe Agrimoniinae. Pollen grains of tribe Sanguisorbeae were generally subprolate to spheroidal in shape, had operculate or pontoperculate apertures, and had three apertures, except for Margyricarpus (tetraperturate). Exine sculpturing within Sanguisorbinae represented variations of striate, verrucate, rugulate, and perforate patterns often with microechinate sculpturing. Striate exine patterns and prolate shapes characterized the pollen of the Agrimoniinae, except for the microechinate-verrucate pattern and subprolate to spheroidal shapes observed in Hagenia. Pollen characters are most useful at the generic level and, when mapped on to a molecular phylogenetic tree of the tribe, are concordant with a monophyletic Agrimoniinae and a clade comprising Margyricarpus + Acaena + Polylepis + Cliffortia + Sanguisorba in the Sanguisorbinae. Outgroup comparison indicated that operculate colpi, three apertures, and polymorphism for striate or microverrucate exines represented primitive states for tribe Sanguisorbeae.




Similar content being viewed by others
References
Byatt JJ (1976) Pollen morphology of some European species of Crataegus L. and of Mespilus germanica L. (Rosaceae). Pollen Spores 18:335–349
Chissoe WB, Skvarla JJ (1996) Combining sputter coating with OTOTO treatment to eliminate charging artifacts in pollen preparations. Proc Okla Acad Sci 76:83–85
Chissoe WB, Vezey EL, Skvarla JJ (1994) Hexamethyldisilazane as a drying agent for pollen scanning electron microscopy. Biotechno Histochem 69:192–198
Chissoe WB, Vezey EL, Skvarla JJ (1995) The use of osmium-thiocarbohydrazide for structural stabilization and enhancement of secondary electron images in scanning electron microscopy of pollen. Grana 34:317–324
Chung K-S (2008) A systematic study of the genus Agrimonia (Rosaceae). Dissertation, University of Oklahoma, Norman
Cooper RL, Osborn JM, Philbrick CT (2000) Comparative pollen morphology and ultrastructure of the Callitrichaceae. Amer J Bot 87:161–175
Eide F (1981) Key for Northwest European Rosaceae pollen. Grana 20:101–118
Erdtman G (1960) The acetolysis method. A revised description. Sven Bot Tidskr 54:561–564
Erdtman G (1963) Palynology. In: Turrill WB (ed) Vistas in botany, vol 4. Pergamon Press, New York, pp 23–54
Erdtman G (1971) Pollen morphology and plant taxonomy. Angiosperms (an introduction to Palynology I). Hafner Publishing Company, New York
Eriksson T, Hibbs MS, Yoder AD, Delwiche CF, Donoghue MJ (2003) The phylogeny of Rosoideae (Rosaceae) based on Sequences of the internal transcribed spacer (ITS) of nuclear ribosomal DNA and the trnL/F region of chloroplast DNA. Int J Pl Sci 164:197–211
Fogle HW (1977a) Identification of clones within four tree fruit species by pollen exine patterns. J Amer Soc Hort Sci 102:552–560
Fogle HW (1977b) Identification of tree fruit species by pollen ultrastructure. J Amer Soc Hort Sci 102:548–551
Hebda RJ, Chinnappa CC (1990a) Pollen morphology of the Rosaceae of western Canada. III. Geum. Can J Bot 68:1369–1378
Hebda RJ, Chinnappa CC (1990b) Studies on pollen morphology of Rosaceae in Canada. Rev Palaeobot Palynol 64:103–108
Hebda RJ, Chinnappa CC (1994) Studies on pollen morphology of Rosaceae. Acta Bot Gallica 141:183–193
Hebda RJ, Chinnappa CC, Smith BM (1988a) Pollen morphology of the Rosaceae of Western Canada I. Agrimonia to Crataegus. Grana 27:95–113
Hebda RJ, Chinnappa CC, Smith BM (1988b) Pollen morphology of the Rosaceae of western Canada. II. Dryas, Fragaria, Holodiscus. Can J Bot 66:595–612
Hebda RJ, Chinnappa CC, Smith BM (1991) Pollen morphology of the Rosaceae of western Canada. IV. Luetkea, Oemleria, Physocarpus, Prunus. Can J Bot 69:2583–2596
Helfgott DM, Francisco-Ortega J, Santos-Guerra A, Jansen RK, Simpson BB (2000) Biogeography and breeding system evolution of the woody Bencomia Alliance (Rosaceae) in Macaronesia based on ITS sequence data. Syst Bot 25:82–97
Hutchinson JJ (1964) The genera of Flowering Plants (Angiospermae), vol I. Oxford University Press, London
Kalkman C (1988) The phylogeny of the Rosaceae. Bot J Linn Soc 98:37–59
Kalkman C (2004) Rosaceae. In: Kubitzki K (ed) The families and genera of vascular plants, vol 6. Flowering plants––dicotyledons: Celastrales, Oxalidales, Rosales, Cornales, Ericales. Springer, Berlin, pp 343–386
Li F, Wan P, Gao S, Ding Y, Zhou F (1999) Pollen morphology identification of five species of medicinal Sanguisorba with electron microscope. Chin J Chin Materia Med 24:715–717
Maas J (1977) Pollen ultrastructure of strawberry and other small-fruit crops. J Amer Soc Hort Sci 102:560–571
Moore PD, Webb JA, Collinson M (1991) Pollen analysis. Blackwell, London
Morgan DR, Soltis DE, Robertson KR (1994) Systematic and evolutionary implications or rbcL sequence variation in Rosaceae. Amer J Bot 81:890–903
Naruhashi N, Toyoshima Y (1979) Pollen morphology of Japanese Rosaceae. J Phytogeogr Taxon 27:46–50
Nordborg G (1966) Sanguisorba L., Sarcopoterium Spach, and Bencomia Webb et Berth. Delimitation and Subdivision of the Genera. Opera Bot 11:1–103
Nordborg G (1967) The genus Sanguisorba section Poterium: experimental studies and taxonomy. Lund Opera Bot 16:1–153
Pérez De Paz J (2004) Rosaceae–Sanguisorbeae de Macaronesia: Generos Marcetella, Bencomia y Dendriopoterium. Palinologia, Biogeografía, Sistemas Sexuales y Filogenia. Bot Macaronesica 25:95–126
Potter D (2003) Molecular phylogenetic studies in Rosaceae. In: Sharma AK, Sharma A (eds) Plant genome: biodiversity and evolution, vol I, Pt. A: Phanerogams. Science Publishers, Inc., Enfield, pp 319–351
Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, Campbell CS (2007) Phylogeny and classification of Rosaceae. Pl Syst Evol 266:5–43
Punt W, Blackmore S, Nilsson S, Le Thomas A (1994) Glossary of pollen and spore terminology. LPP Foundation, Utrecht
Reitsma T (1966) Pollen morphology of some European Rosaceae. Acta Bot Neerl 15:290–307
Reitsma T (1967) Some aspects of the pollen morphology of genus Sanguisorba L. (Rosaceae). Rev Palaeobot Palynol 4:305–310
Schulze-Menz GK (1964) Rosaceae. In: Melchior H (ed) Engler’s Syllabus der Pflanzenfamilien II, 12th edn. Gebrϋ der Borntraeger, Berlin, pp 209–218
Skvarla JJ, Rowley JR, Hoch PC, Chissoe WF (2005) Viscin threads on pollen of Circaea xintermedia Ehrh. (Circaeeae: Onagraceae). Taxon 54:121–126
Wen J, Nowicke JW (1999) Pollen Ultrastructure of Panax (the ginseng genus, Araliaceae), an Eastern Asian and Eastern North American Disjunct Genus. Amer J Bot 86:1624–1636
Wodehouse RP (1935) Pollen grains. McGraw Hill, New York
Acknowledgments
We would like to thank William Chissoe and Greg Strout and the University of Oklahoma Samuel Noble Electron Microscopy Laboratory for help with scanning electron microscopy. Amy Buthod of the Robert Bebb Herbariun (OKL) assisted in acquiring herbarium specimens. Two anonymous reviewers provided valuable assistance in interpreting pollen structures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, KS., Elisens, W.J. & Skvarla, J.J. Pollen morphology and its phylogenetic significance in tribe Sanguisorbeae (Rosaceae). Plant Syst Evol 285, 139–148 (2010). https://doi.org/10.1007/s00606-009-0262-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-009-0262-9