Abstract
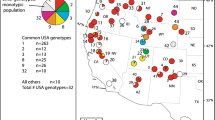
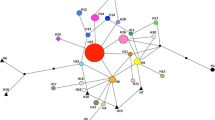
Horticulture is one of the most important pathways for plant invasion. We used microsatellite markers to reveal the impact of plant breeding on Mahonia aquifolium, an invasive ornamental shrub. Since it was bred by hybridization with the related species M. repens and M. pinnata, we compared populations of the three native species, various commercial cultivars and invasive populations. Invasive populations and cultivars were genetically differentiated from the native groups, but differences did not result from genetic bottlenecks. In cultivars but not in invasive populations, we proved genes from M. pinnata. No significant amount of M. repens genes were found in cultivars and invasive populations, but this result has to be viewed with caution because of the close relationship between native M. aquifolium and M. repens. We conclude that the evolution of invasive Mahonia populations was a result of restriction of gene pool during introduction, secondary release, and artificial selection, in combination with an increase of genetic diversity by plant breeders and by extensive gene flow.




Similar content being viewed by others
References
Ahrendt LWA (1961) Berberis and Mahonia. A taxonomic revision. Bot J Linn Soc Lond 57:1–410
Allendorf FW, Lundquist LL (2003) Introduction: Population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Auge H, Brandl R (1997) Seedling recruitment in the invasive clonal shrub, Mahonia aquifolium Pursh (Nutt.). Oecologia 110:205–211
Barrett SCH, Richardson BJ (1986) Genetic attributes of invading species. In: Groves RH, Burdon JJ (eds) Ecology of biological invasions. Cambridge University Press, Cambridge, pp 21–33
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Bundesverband Deutscher Pflanzenzüchter e.V. (2007) Aufgaben der Pflanzenzüchtung. http://www.bdp-online.de/zuechtung/zuecht1.php?menu=3
Dehnen-Schmutz K, Touza J, Perrings C, Williamson M (2007) The horticultural trade and ornamental plant invasions in Britain. Conserv Biol 21:224–231
Dieringer D, Schlötterer C (2002) Microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Molec Ecol Notes 3:167–169
Durka W, Bossdorf O, Prati D, Auge H (2005) Molecular evidence for multiple introduction of garlic mustard (Alliaria petiolata, Brassicaceae) to North America. Molec Ecol 14:1697–1706
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants. Proc Natl Acad Sci USA 97:7043–7050
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Molec Ecol 14:2611–2620
Goudet J (1995) FSTAT (Version 1.2): A computer program to calculate F- statistics. J. Heredity 86:485–486
Goudet J (1999) PCAGEN. http://www2.unil.ch/popgen/softwares/pcagen.htm
Gray AJ (1986) Do invading species have definable genetic characteristics? Phil Trans Roy Soc Lond 314:655–674
Günther H (1979) Schöne Blütengehölze. VEB Deutscher Landwirtschaftsverlag DDR, Berlin
Hollingsworth ML, Hollingsworth PM, Jenkins GI, Bailey JP, Ferris C (1998) The use of molecular markers to study patterns of genotypic diversity in some invasive alien Fallopia spp. (Polygonaceae). Molec Ecol 7:1681–1691
Houtman RT, Kraan KJ, Kromhout H (2004) Mahonia aquifolium, M. repens, M. x wagneri en hybriden. Dendroflora 41:42–69
Kim Y-D, Kim S-H, Landrum LR (2004) Taxonomic and phytogeographic implications from ITS phylogeny in Berberis (Berberidaceae). J Pl Res 117:175–182
Kitajima K, Fox AM, Sato T, Nagamatsu D (2006) Cultivar selection prior to introduction may increase invasiveness: evidence from Ardisia crenata. Biol Invas 8:1471–1482
Klotz S, Kühn I, Durka W (2002) BIOLFLOR - Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland. Schriftenreihe für Vegetationskunde, vol 38, Bonn—Bad Godesberg
Kowarik I (1992) Einführung und Ausbreitung nichteinheimischer Gehölzarten in Berlin und Brandenburg. Verhandlungen Botanischer Vereine Berlin Brandenburg 3:1–188
Kowarik I (1995) Time lags in biological invasions with regard to the success and failure of alien species. In: Pysek P, Prach K, Rejmanek M, Wade M (eds) Plant invasions—general aspects and special problems. Academic Publishing, Amsterdam, pp 15–38
Kowarik I (2005) Urban ornamentals escaped from cultivation. In: Gressel J (ed) Crop Ferality and Volunteerism. CRC Press, Boca Raton, pp 97–121
Laferriere J (1997) Transfer of specific and infraspecific taxa from Mahonia to Berberis (Berberidaceae). Bot Zhurn 82:95–99
Lodge DM (1993) Biological Invasions: Lessons for Ecology. Trends Ecol Evol 8:133–137
Mack RN (2000) Cultivation fosters plant naturalization by reducing environmental stochasticity. Biol Invas 2:111–122
Maron JL, Vila M, Bommarco R, Elmendorf S, Beardsley P (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280
Milne RI, Abbott RJ (2000) Origin and evolution of invasive naturalized material of Rhododendron ponticum L. in the British Isles. Molec Ecol 9:541–556
Monzingo HN (1987) Shrubs of the Great Basin. University of Nevada Press, Reno
Munz P (1959) A California flora. University of California Press, Berkeley
Neuffer B, Auge H, Mesch H, Amarell U, Brandl R (1999) Spread of violets in polluted pine forests: morphological and molecular evidence for the ecological importance of interspecific hybridization. Molec Ecol 8:365–377
Okada M, Ahmad R, Jasieniuk M (2007) Microsatellite variation points to local landscape plantings as sources of invasive pampas grass (Cortaderia selloana) in California. Molec Ecol 16:4956–4971
Perrins J, Fitter A, Williamson M (1993) Population biology and rates of invasion of three introduced Impatiens species in the British Isles. J Biogeogr 20:33–44
Piper CV (1906) Flora of the state of Washington. Contr US Natl Herb 11:282–283
Piper CV (1922) The identification of Berberis aquifolium and Berberis repens. Contr US Natl Herb 20:437–451
Preston CD, Telfer MG, Arnold HR, Carey PD, Cooper JM, Dines TD, Hill MO, Pearman DA, Roy DB, Smart SM (2002) The changing flora of the UK. DEFRA, London
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pysek P, Prach K, Smilauer P (1995) Relating invasion success to plant traits: An analysis of the Czech alien flora. In: Pysek P, Prach K, Rejmanek M, Wade M (eds) Plant invasions—general aspects and special problems. SPB Academic Publishing, Amsterdam, pp 39–60
Rejmanek M (2000) Invasive plants: approaches and predictions. Austral Ecol 25:497–506
Rejmanek M (1996) A theory of seed plant invasiveness: The first sketch. Biol Conserv 78:171–181
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993
Rosenberg NA (2004) DISTRUCT: a program for the graphical display of population structure. Mol Ecol Notes 4:137–138
Roß C, Durka W (2006) Isolation and characterization of microsatellite markers in the invasive shrub Mahonia aquifolium (Berberidaceae) and their applicability in related species. Molec Ecol Notes 6:948–950
Schneider S, Roessli D, Excoffier L (2000) Arlequin Version (2.000): a software for population genetics data analysis
Soldaat LL, Auge H (1998) Interactions between an invasive plant, Mahonia aquifolium, and a native phytophagous insect, Rhagoletis meigenii. In: Starfinger U, Edwards K, Kowarik I, Williamson M (eds) Plant invasions: ecological mechanisms and human responses. Backhuys Publishers, Leiden, pp 347–360
Torrey J, Gray A (1838) Berberidaceae. In: Flora of North America. Oxford University Press, Oxford
van de Laar HJ (1975) Mahonia en Mahoberberis. Dendroflora 11(12):19–33
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population-structure. Evolution 38:1358–1370
Whittemore AT (1997) Berberis. In: Flora of North America. Oxford University Press, New York, pp 276–286
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Acknowledgments
We thank Verena Schmidt, Christina Belle, Thomas Bunge, Vera Draba, Michael Beckmann, Claudia Paschke and Dorit Raudnitschka for their help in collecting berries and rearing seedlings. Further on, we thank Klaus Hempel and Antje Thondorf for their technical support and Ina Geier and Martina Herrmann for help in the laboratory. We thank all colleagues who gave us comments on invasive Mahonia populations and thanks to all those who sampled seeds, notably Alisa Ramakrishnan, Daniel Jones, Daniel Prati, David W. Inouye, Heather Davis, Jennifer Williams, Mark Schwarzlaender, Mark van Kleunen, Mary Bricker, Petr Pysek, Rosemarie De Clerck-Floate, Ruth Hufbauer, Shanna Carney and Steven Rauth. Many thanks to several botanical gardens and tree nurseries which provided cultivars and many thanks to Hans-Joachim Albrecht, Heribert Reif, Wilfried Müller-Platz and Wout Kromhout for their support and information about Mahonia cultivars. The study was financially supported by the BioTeam program of the German Federal Ministry of Education and Research, project ID 01LM0206.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ross, C.A., Auge, H. & Durka, W. Genetic relationships among three native North-American Mahonia species, invasive Mahonia populations from Europe, and commercial cultivars. Plant Syst Evol 275, 219–229 (2008). https://doi.org/10.1007/s00606-008-0066-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0066-3