Abstract
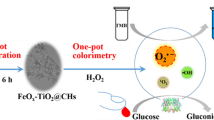
By employing NH2-MIL-88 as a template, we synthesized the intermediate Fe@CN under high-temperature calcination and further fabricated the FeS2@CN nanocomposites in the presence of sulfur powder. Under varying temperatures (300–600 °C) and Fe@CN-to-S ratios (1:3–6), FeS2@CN500-5 nanocomposites had the highest peroxidase-mimetic activity. Under optimized conditions (incubation temperature 40 °C; solution pH 4.0 and nanocomposite concentration 10 μg/mL; 652-nm absorption), the Michaelis-Menten constant (Km) of FeS2@CN was much lower than that of horseradish peroxidase (HRP), therefore demonstrating that it had a higher affinity for both chromogenic substrates than conventional HRP. The limits of detection for H2O2 and glucose were 0.15 and 0.30 μmol/L, respectively, and the recoveries for glucose were 91.8–103% with RSDs <5.2%. The novelty of this study lies in (1) the FeS2@CN was confirmed to possess stronger enzyme-mimetic activity than its precursors (NH2-MIL-88 and Fe@CN); (2) the enhanced activity resulted from the unsaturated sites of N and S doping and the plentiful defects on the porous carbon surface; and (3) free radical trapping experiments evidenced that •OH played a major role in the catalytic reaction, while h+ and •O2− simultaneously participated in the catalytic process. These convincing performance metrics lead us to postulate that the FeS2@CN-based colorimetric biosensor provides a promising approach for several real-world applications, such as point-of-care diagnosis and workplace health evaluations.
Graphical abstract





Similar content being viewed by others
References
Bhadra BN, Ahmed I, Kim S et al (2017) Adsorptive removal of ibuprofen and diclofenac from water using metal-organic framework-derived porous carbon. Chem Eng J 314:50–58. https://doi.org/10.1016/j.cej.2016.12.127
Deng H, Doonan CJ, Furukawa H et al (2010) Multiple functional groups of varying ratios in metal-organic frameworks. Science 327:846–850. https://doi.org/10.1126/science.1181761
Tranchemontagne DJ, Mendoza-Cortés JL, O’Keeffe M et al (2009) Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem Soc Rev 38:1257–1283. https://doi.org/10.1039/b817735j
Garibay SJ, Wang Z, Tanabe KK et al (2009) Postsynthetic modification: a versatile approach toward multifunctional metal-organic frameworks. Inorg Chem 48:7341–7349. https://doi.org/10.1021/ic900796n
Li Y, Wang LJ, Fan HL et al (2015) Removal of sulfur compounds by a copper-based metal organic framework under ambient conditions. Energ Fuel 29:298–304. https://doi.org/10.1021/ef501918f
Li JR, Sculley J, Zhou HC (2012) Metal-organic frameworks for separations. Chem Rev 112:869–932. https://doi.org/10.1002/adma.201705189
Farha OK, Spokoyny AM, Hauser BG et al (2009) Synthesis, properties, and gas separation studies of a robust diimide-based microporous organic polymer. Chem Mater 21:3033–3035. https://doi.org/10.1002/adma.201705189
Qi Z, Wang L, You Q et al (2017) PA-Tb-Cu MOF as luminescent nanoenzyme for catalytic assay of hydrogen peroxide. Biosens Bioelectron 96:227–232. https://doi.org/10.1016/j.bios.2017.05.013
Fu Y, Zhang H, Dai S et al (2015) Glutathione-stabilized palladium nanozyme for colorimetric assay of silver (I) ions. Analyst 140:6676–6683. https://doi.org/10.1039/C5AN01103E
Liu S, Lu F, Xing R et al (2011) Structural effects of Fe3O4 nanocrystals on peroxidase-like activity. Chem Eur J 17:620–625. https://doi.org/10.1002/chem.201001789
Kim JD, Kim M, Kong L et al (2018) Self-anchored catalyst interface enables ordered via array formation from submicrometer to millimeter scale for polycrystalline and single-crystalline silicon. ACS Appl Mater Inter 10:9116–9122. https://doi.org/10.1021/acsami.7b17708
Liu Y, Wang C, Yang H et al (2015) Uniformloaded SnS2/Single-walled carbon nanotubes hybrid with improved electrochemical performance for lithium-ion battery. Mater Lett 159:329–332. https://doi.org/10.1016/j.matlet.2015.07.036
Yu S, Jung JW, Kim ID et al (2015) Single layers of WS2 nanoplates embedded in nitrogen-doped carbon nanofibers as anode materials for lithium-ion batteries. Nanoscale 7:11945–11950. https://doi.org/10.1039/c5nr02425k
Xu X, Fan Z, Yu X et al (2014) A nanosheets-on-channel architecture constructed from MoS2 and CMK-3 for high-capacity and long-cycle-life lithium storage. Adv Energy Mater 4:1400902. https://doi.org/10.1002/aenm.201400902
Shao M, Cheng Y, Zhang T et al (2018) Designing MOFs-derived FeS2@carbon composites for high-rate sodium ion storage with capacitive contributions. ACS Appl Mater Inter 10:33097–33104. https://doi.org/10.1021/acsami.8b10110
Sun C, Gradzielski M (2021) Upconversion-based nanosystems for fluorescence sensing of pH and H2O2. Nanoscale Adv 3:2538–2546. https://doi.org/10.1039/d0na01045f
Xie BB, Yang XH, Zhang RX (2021) Hollow and porous Fe3C-NC nanoballoons nanozymes for cancer cell H2O2 detection. Sensor Actuat B Chem 347:130597. https://doi.org/10.1016/j.snb.2021.130597
Cole JB, Florez JC (2020) Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 16:377–390. https://doi.org/10.1038/s41581-020-0278-5
Ning D, Liu Q (2019) Luminescent MOF nanosheets for enzyme assisted detection of H2O2 and glucose and activity assay of glucose oxidase-ScienceDirect. Sensor Actuat B Chem 282:443–448. https://doi.org/10.1016/j.snb.2018.11.088
Zhu N, Zou Y, Huang M et al (2018) A sensitive colorimetric immunosensor based on Cu-MOFs and HRP for detection of dibutyl phthalate in environmental and food samples. Talanta 186:104–109. https://doi.org/10.1016/j.talanta.2018.04.023
Zhu XX, Xue Y, Han S et al (2020) V2O5-montmorillonite nanocomposites of peroxidase-like activity and their application in the detection of H2O2 and glutathione. Appl Clay Sci 195:105718. https://doi.org/10.1016/j.clay.2020.105718
Hosseini M, Sabet FS, Kahbbaz H et al (2017) Enhancement of peroxidase-like activity of cerium-doped ferrite nanoparticle for colorimetric detection of H2O2 and glucose. Anal methods 9:3519–3524. https://doi.org/10.1039/c7ay00750g
Lv SW, Zhao N, Liu JM et al (2021) Newly constructed NiCo2O4 derived from ZIF-67 with dual mimic enzyme properties for colorimetric detection of biomolecules and metal ions. ACS Appl Mater Inter 13:25044–25052. https://doi.org/10.1021/acsami.1c06705
Raizada P, Khan A, Singh P et al (2020) Construction of carbon nanotube mediated Fe doped graphitic carbon nitride and Ag3VO4 based Z-scheme heterojunction for H2O2 assisted 2,4 dimethyl phenol photodegradation. Sep Purif Technol 247:116957. https://doi.org/10.1016/j.seppur.2020.116957
Jin X, Gao S, Wu A et al (2020) Dual-constrained sulfur in FeS2@C nanostructured lithium-sulfide batteries. ACS Appl Energ Mater 3:10950–10960. https://doi.org/10.1021/acsaem.0c01929
Se-Na K, Gwon PC, Kang HB et al (2018) Metal-organic frameworks NH2-MIL-88(Fe) as carriers for ophthalmic delivery of brimonidine. Acta Biomater 79:344–353. https://doi.org/10.1016/j.actbio.2018.08.023
Liu W, Wang YY, Ai ZH et al (2015) Hydrothermal synthesis of FeS2 as a high-efficiency fenton reagent to degrade alachlor via superoxide-mediated Fe(II)/Fe(III) cycle. ACS Appl Mater Inter 7:28534–28544. https://doi.org/10.1021/acsami.5b09919
Liang Y, Bai P, Zhou J et al (2016) An efficient precursor to synthesize various FeS2 nanostructures via simple hydrothermal synthesis method. CrystEngComm 10:6262–6271. https://doi.org/10.1039/c6ce01203e
Wang XX, Wu Q, Shan Z et al (2011) BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens Bioelectro 26:3614–3619. https://doi.org/10.1016/j.bios.2011.02.014
Hu L, Yuan Y, Zhang L et al (2013) Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta 762:83–86. https://doi.org/10.1016/j.aca.2012.11.056
He YL, Li N, Liu XW et al (2021) 5,10,15,20-tetrakis (4-carboxyl phenyl) porphyrin-functionalized urchin-like CuCo2O4 as an excellent artificial nanozyme for determination of dopamine. Microchim Acta 188:1–11. https://doi.org/10.1007/s00604-021-04819-9
He YL, Li N, Li WK et al (2020) 5,10,15,20-tetrakis (4-carboxylphenyl) porphyrin functionalized NiCo2S4 yolk-shell nanospheres: excellent peroxidase-like activity, catalytic mechanism and fast cascade colorimetric biosensor for cholesterol-Science direct. Sensor Actuat B Chem 326:128850. https://doi.org/10.1016/j.snb.2020.128850
Shi W, Zhang X, He S et al (2011) CoFe2O4 magnetic nanoparticles as a peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem Commun 47:10785–10787. https://doi.org/10.1039/c1cc14300j
Gao L, Wu J, Gao D (2011) Enzyme-controlled self-assembly and transformation of nanostructures in a tetramethylbenzidine/horseradish peroxidase/H2O2 system. ACS Nano 5:6736–6742. https://doi.org/10.1021/nn2023107
Lu J, Zhang H, Li S et al (2020) Oxygen-vacancy-enhanced peroxidase-like activity of reduced Co3O4 nanocomposites for the colorimetric detection of H2O2 and glucose. Inorg Chem 59:3152–3159. https://doi.org/10.1021/acs.inorgchem.9b03512
Cai S, Han Q, Qi C et al (2015) Pt74Ag26 nanoparticle-decorated ultrathin MoS2 nanosheets as novel peroxidase mimics for highly selective colorimetric detection of H2O2 and glucose. Nanoscale 8:3685–3693. https://doi.org/10.1039/c5nr08038j
Hai G, Yang P, Zhang YM et al (2017) A bimetallic (Co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim Acta 184:4629–4635. https://doi.org/10.1007/s00604-017-2509-4
Wu YZ, Ma YJ, Xu GH et al (2017) Metal-organic framework coated Fe3O4 magnetic nanoparticles with peroxidase-like activity for colorimetric sensing of cholesterol. Sensor Actuat B Chem 249:195–202. https://doi.org/10.1016/j.snb.2017.03.145
Jin LH, Shang L, Guo S et al (2011) Biomolecule-stabilized Au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens and Bioelectron 26:1965–1969. https://doi.org/10.1016/j.bios.2010.08.019
Funding
This work was jointly supported by the National Science Foundation of China (22076134 and 21876125), Zhejiang Provincial Public Benefit Project (LGC22B070002), Jiangsu Provincial Natural Science Foundation (BK20211338), Key Science & Technology Project of Suzhou City (SS202028), Zhejiang Provincial University Student Sci&Tech Innovation Activity Plan and New Seedling Talent Plan (No. 2021R413061), and National Training Program of Innovation and Entrepreneurship for Undergraduates (202110343068S).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, R., Tian, J., Wang, H. et al. Sensitive colorimetric assay of hydrogen peroxide and glucose in humoral samples based on the enhanced peroxidase-mimetic activity of NH2-MIL-88-derived FeS2@CN nanocomposites compared to its precursors. Microchim Acta 189, 427 (2022). https://doi.org/10.1007/s00604-022-05525-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05525-w