Abstract
Mitochondria, as the energy factory of most cells, are not only responsible for the generation of adenosine triphosphoric acid (ATP) but also essential targets for therapy and diagnosis of various diseases, especially cancer. The safe and potential nanoplatform which can deliver various therapeutic agents to cancer cells and mitochondrial targeted imaging is urgently required. Herein, Au nanoparticles (AuNPs), mesoporous silica nanoparticles (MSN), cationic ligand (triphenylphosphine (TPP)), doxorubicin (DOX), and carbon nanodots (CDs) were utilized to fabricate mitochondrial targeting drug delivery system (denoted as CDs(DOX)@MSN-TPP@AuNPs). Since AuNPs, as the gatekeepers, can be etched by intracellular glutathione (GSH) via ligand exchange induced etching process, DOX can be released into cells in a GSH-dependent manner which results in the superior GSH-modulated tumor inhibition activity. Moreover, after etching by GSH, the CDs(DOX)@MSN-TPP@AuNPs can serve as promising fluorescent probe (λex = 633 nm, λem = 650 nm) for targeted imaging of mitochondria in living cells with near-infrared fluorescence. The induction of apoptosis derived from the membrane depolarization of mitochondria is the primary anti-tumor route of CDs(DOX)@MSN-TPP@AuNPs. As a kind of GSH-responsive mitochondrial targeting nanoplatform, it holds great promising for effective cancer therapy and mitochondrial targeted imaging.
Graphical abstract

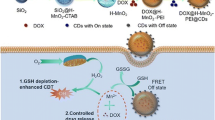
The mitochondrial targeting drug delivery system was fabricated by AuNPs, MSN, TPP, and CDs. The nanoplatform can realize redox-responsive drug delivery and targeted imaging of mitochondria in living cells to improve the therapeutic efficiency and security.







Similar content being viewed by others
References
Ping Y, Guo J, Ejima H, Chen X, Richardson JJ, Sun H, Caruso F (2015) pH-responsive capsules engineered from metal–phenolic networks for anticancer drug delivery. Small 11(17):2032–2036
Huang JG, Leshuk T, Gu FX (2011) Emerging nanomaterials for targeting subcellular organelles. Nano Today 6(5):478–492
Torchilin VP, Khaw B-A, Weissig V (2002) Intracellular targets for DNA delivery: nuclei and mitochondria. Somat Cell Mol Genet 27(1):49–64
Xu Z, Chen X, Sun Z, Li C, Jiang B (2019) Recent progress on mitochondrial targeted cancer therapy based on inorganic nanomaterials. Mater Today Chem 12:240–260
Indran IR, Tufo G, Pervaiz S, Brenner C (2011) Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta 1807(6):735–745
Jones D (2008) End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov 7(12):961–962
Galluzzi L, Larochette N, Zamzami N, Kroemer G (2006) Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene 25(34):4812–4830
Yingchoncharoen P, Kalinowski DS, Richardson DR (2016) Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev 68(3):701–787
Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z (2011) Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release 152(1):2–12
Qu Q, Ma X, Zhao Y (2016) Anticancer effect of α-tocopheryl succinate delivered by mitochondria-targeted mesoporous silica nanoparticles. ACS Appl Mater Interfaces 8(50):34261–34269
Qu Q, Ma X, Zhao Y (2015) Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers. Nanoscale 7(40):16677–16686
He Q, Shi J (2014) MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Adv Mater 26(3):391–411
Chen M, He X, Wang K, He D, Yang S, Qiu P, Chen S (2014) A pH-responsive polymer/mesoporous silica nano-container linked through an acid cleavable linker for intracellular controlled release and tumor therapy in vivo. J Mater Chem B 2(4):428–436
Yang G, Chen C, Zhu Y, Liu Z, Xue Y, Zhong S, Wang C, Gao Y, Zhang W (2019) GSH-activatable NIR nanoplatform with mitochondria targeting for enhancing tumor-specific therapy. ACS Appl Mater Interfaces 11(48):44961–44969
Jomova K, Vondrakova D, Lawson M, Valko M (2010) Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345(1):91–104
Hong R, Han G, Fernández JM, B-j K, Forbes NS, Rotello VM (2006) Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc 128(4):1078–1079
Zheng M, Liu S, Li J, Qu D, Zhao H, Guan X, Hu X, Xie Z, Jing X, Sun Z (2014) Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv Mater 26(21):3554–3560
Mu J, Lin J, Huang P, Chen X (2018) Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem Soc Rev 47(15):5554–5573
Xu Z, Liu Y (2021) The behavior of carbonized polymer dots at the nano-bio interface and their luminescent mechanism: a physical chemistry perspective. Chin J Chem 39:265–273
Huang Q, Lin X, Li F, Weng W, Lin L, Hu S (2015) Synthesis and applications of carbon dots. Prog Chem 27(11):1604–1614
Pan L, Sun S, Zhang L, Jiang K, Lin H (2016) Near-infrared emissive carbon dots for two-photon fluorescence bioimaging. Nanoscale 8(39):17350–17356
Liu C, Bao L, Tang B, Zhao J-Y, Zhang Z-L, Xiong L-H, Hu J, Wu L-L, Pang D-W (2016) Fluorescence-converging carbon nanodots-hybridized silica nanosphere. Small 12(34):4702–4706
Zhang S, Xiao C, He H, Xu Z, Wang B, Chen X, Li C, Jiang B, Liu Y (2020) The adsorption behaviour of carbon nanodots modulated by cellular membrane potential. Environ Sci Nano 7(3):880–890
Miao X, Qu D, Yang D, Nie B, Zhao Y, Fan H, Sun Z (2018) Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv Mater 30(1):1704740
He W, Kim H-K, Wamer WG, Melka D, Callahan JH, Yin J-J (2014) Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J Am Chem Soc 136(2):750–757
Lu Y, Chen W (2012) Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chem Soc Rev 41(9):3594–3623
Wan L, Chen Z, Deng Y, Liao T, Kuang Y, Liu J, Duan J, Xu Z, Jiang B, Li C (2020) A novel intratumoral pH/redox-dual-responsive nanoplatform for cancer MR imaging and therapy. J Colloid Interface Sci 573:263–277
Liu J, Chang B, Li Q, Xu L, Liu X, Wang G, Wang Z, Wang L (2019) Redox-responsive dual drug delivery nanosystem suppresses cancer repopulation by abrogating doxorubicin-promoted cancer stemness, metastasis, and drug resistance. Adv Sci 6(7):1801987
Zhang M, Liu J, Kuang Y, Li Q, Chen H, Ye H, Guo L, Xu Y, Chen X, Li C, Jiang B (2016) “Stealthy” chitosan/mesoporous silica nanoparticle based complex system for tumor-triggered intracellular drug release. J Mater Chem B 4(19):3387–3397
Zinchuk V, Grossenbacher-Zinchuk O (2009) Recent advances in quantitative colocalization analysis: focus on neuroscience. Prog Histochem Cytochem 44(3):125–172
Ponraj T, Vivek R, Paulpandi M, Rejeeth C, Nipun Babu V, Vimala K, Anand K, Sivaselvam S, Vasanthakumar A, Ponpandian N, Kannan S (2018) Mitochondrial dysfunction-induced apoptosis in breast carcinoma cells through a pH-dependent intracellular quercetin NDDS of PVPylated-TiO2NPs. J Mater Chem B 6(21):3555–3570
Friedman JR, Nunnari J (2014) Mitochondrial form and function. Nature 505(7483):335–343
Zhang X, Yan Q, Mulatihan DN, Zhu J, Fan A, Wang Z, Zhao Y (2018) Pharmaceutical micelles featured with singlet oxygen-responsive cargo release and mitochondrial targeting for enhanced photodynamic therapy. Nanotechnology 29(25):255101
Thangam R, Sathuvan M, Poongodi A, Suresh V, Pazhanichamy K, Sivasubramanian S, Kanipandian N, Ganesan N, Rengasamy R, Thirumurugan R, Kannan S (2014) Activation of intrinsic apoptotic signaling pathway in cancer cells by Cymbopogon citratus polysaccharide fractions. Carbohydr Polym 107:138–150
Funding
This work received financial support from the National Natural Science Foundation of China (22073025, 21603067) and Hubei Nature Science Foundation of China (2019CFB748).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection Nanomaterials for biomedical imaging and targeting
Supplementary information
ESM 1
(DOCX 3489 kb)
Rights and permissions
About this article
Cite this article
He, H., Meng, S., Li, H. et al. Nanoplatform based on GSH-responsive mesoporous silica nanoparticles for cancer therapy and mitochondrial targeted imaging. Microchim Acta 188, 154 (2021). https://doi.org/10.1007/s00604-021-04810-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04810-4