Abstract
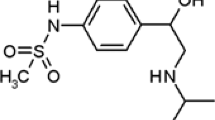
An ultrasonic-assisted dispersive solid-phase microextraction was developed by a multi-stimuli responsive molecularly imprinted polymer based on chain transfer agent-modified chitosan nanoparticles for enrichment and separation of trace capecitabine in a real sample. The synthesized particles were carefully characterized and it was found that a uniform pH-sensitive imprinted polymeric shell is obtained on the surface of Fe3O4@chitosan core with enough saturation magnetization (29 emu g−1). Desirable adsorption capacity (91 mg g−1) and high imprinting factor (IF = 3.6) toward capecitabine were exhibited by the Langmuir isotherm model. Under optimized conditions, which were achieved by experimental design, the detection limit (S/N = 3) was 1.9 ng mL−1. The obtained relative mean recoveries of capecitabine using high-performance liquid chromatography were in the range of 93 to 102% in a human plasma sample with RSD less than 5.5%.

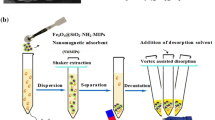
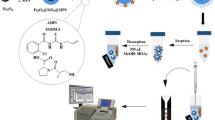
Schematic representation of ultrasonic-assisted dispersive solid-phase microextraction by multi-stimuli responsive molecularly imprinted polymer based on chain transfer agent-modified chitosan nanoparticles and high-performance liquid chromatography for enrichment and separation of capecitabine in the real sample.



Similar content being viewed by others
References
Dudhipala N, Puchchakayala G (2018) Capecitabine lipid nanoparticles for anti-colon cancer activity in 1,2-dimethylhydrazine-induced colon cancer: preparation, cytotoxic, pharmacokinetic, and pathological evaluation. Drug Dev Ind Pharm 44:1572–1582
Pacheco JG, Silva MSV, Freitas M, Nouws HPA, Delerue-Matos C (2018) Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3). Sensors Actuators B Chem 256:905–912
Forough M, Farhadi K, Molaei R, Khalili H, Shakeri R, Zamani A, Matin AA (2017) Capillary electrophoresis with online stacking in combination with AgNPs@MCM-41 reinforced hollow fiber solid-liquid phase microextraction for quantitative analysis of Capecitabine and its main metabolite 5-fluorouracil in plasma samples isolated from cancer patients. J Chromatogr B 1040:22–37
Thorat SG, Chikhale RV, Tajne MR (2018) A rapid and simple HPTLC assay for therapeutic drug monitoring of capecitabine in colorectal cancer patients. Biomed Chromatogr:32. https://doi.org/10.1002/bmc.4100 Epub 2017 Oct 15
Deenen MJ, Rosing H, Hillebrand MJ, Schellens JHM, Beijnen JH (2013) Quantitative determination of capecitabine and its six metabolites in human plasma using liquid chromatography coupled to electrospray tandem mass spectrometry. J Chromatogr B 913-914:30–40
Dhananjeyan MR, Liu J, Bykowski C, Trendel JA, Sarver JG, Ando H, Erhardt PW (2007) Rapid and simultaneous determination of capecitabine and its metabolites in mouse plasma, mouse serum, and in rabbit bile by high-performance liquid chromatography. J Chromatogr A 1138:101–108
Zufía L, Aldaz A, Giráldez J (2004) Simple determination of capecitabine and its metabolites by liquid chromatography with ultraviolet detection in a single injection. J Chromatogr B 809:51–58
Deng P, Ji C, Dai X, Zhong D, Ding L, Chen X (2015) Simultaneous determination of capecitabine and its three nucleoside metabolites in human plasma by high performance liquid chromatography–tandem mass spectrometry. J Chromatogr B 989:71–79
Siethoff C, Orth M, Ortling A, Brendel E, Wagner-Redeker W (2004) Simultaneous determination of capecitabine and its metabolite 5-fluorouracil by column switching and liquid chromatographic/tandem mass spectrometry. J Mass Spectrom 39:884–889
Licea-Perez H, Wang S, Bowen CL, Licea-Perez H (2009) Development of a sensitive and selective LC-MS/MS method for the determination of α-fluoro-β-alanine, 5-fluorouracil and capecitabine in human plasma. J Chromatogr B 877:1040–1046
Kalanur SS, Seetharamappa J, Mamatha GP, Hadagali MD, Kandagal PB (2008) Electrochemical behavior of an anti-cancer drug at glassy carbon electrode and its determination in pharmaceutical formulations. Int J Electrochem Sci 3:756–767
Ansari S, Masoum S (2018) A multi-walled carbon nanotube-based magnetic molecularly imprinted polymer as a highly selective sorbent for ultrasonic-assisted dispersive solid-phase microextraction of sotalol in biological fluids. Analyst 143:2862–2875
Pastor-Belda M, Garrido I, Campillo N, Viñas P, Hellín P, Flores P, Fenoll J (2016) Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid–liquid microextraction. Food Chem 202:389–395
Ansari S, Karimi M (2017) Novel developments and trends of analytical methods for drug analysis in biological and environmental samples by molecularly imprinted polymers. Trends Anal Chem 89:146–162
Ansari S, Masoum S (2019) Molecularly imprinted polymers for capturing and sensing proteins: current progress and future implications. Trends Anal Chem 114:29–47
Takeuchi T, Kitayama Y, Sasao R, Yamada T, Toh K, Matsumoto Y, Kataoka K (2017) Molecularly imprinted nanogels acquire stealth in situ by cloaking themselves with native dysopsonic proteins. Angew Chem Int Ed 56:7088–7092
Wei Y, Zeng Q, Hu Q, Wang M, Tao J, Wang L (2018) Self-cleaned electrochemical protein imprinting biosensor basing on a thermo-responsive memory hydrogel. Biosens Bioelectron 99:136–141
Wang HS, Song M, Hang TJ (2016) Functional interfaces constructed by controlled/living radical polymerization for analytical chemistry. ACS Appl Mater Interfaces 8:2881–2898
Qu J, Liu G, Wang Y, Hong R (2010) Preparation of Fe3O4–chitosan nanoparticles used for hyperthermia. Adv Powder Technol 21:461–467
Jiang J, Pan X, Cao J, Jiang J, Hua D, Zhu X (2012) Synthesis and property of chitosan graft copolymer by RAFT polymerization with tosylic acid–chitosan complex. Int J Biol Macromol 50:586–590
He Y, Huang Y, Jin Y, Liu X, Liu G, Zhao R (2014) Well-defined nanostructured surface-imprinted polymers for highly selective magnetic separation of fluoroquinolones in human urine. ACS Appl Mater Interfaces 6:9634–9642
Ansari S, Karimi M (2017) Recent configurations and progressive uses of magnetic molecularly imprinted polymers for drug analysis. Talanta 167:470–485
Asfaram A, Ghaedi M, Dashtian K (2017) Ultrasound assisted combined molecularly imprinted polymer for selective extraction of nicotinamide in human urine and milk samples: spectrophotometric determination and optimization study. Ultrason Sonochem 34:640–650
Abdollahi E, Abdouss M, Mohammadi A (2016) Synthesis of a nano molecularly imprinted polymeric sorbent for solid phase extraction and determination of phenytoin in plasma, urine, and wastewater by HPLC. RSC Adv 6:39095–39105
Attallah OA, Al-Ghobashy MA, Ayoub AT, Tuszynski JA, Nebsen M (2018) Computer-aided design of magnetic molecularly imprinted polymer nanoparticles for solid-phase extraction and determination of levetiracetam in human plasma. RSC Adv 8:14280–14292
An LJ, Wang J, Pang ZY, Xi RM (2014) Magnetic molecularly imprinted silica gel for enrofloxacin recognition. Colloids Surf A Physicochem Eng Asp 452:125–128
Pan J, Zou X, Wang X, Guan W, Yan Y, Han J (2010) Selective recognition of 2,4-dichlorophenol from aqueous solution by uniformly sized molecularly imprinted microspheres with β-cyclodextrin/attapulgite composites as support. Chem Eng J 162:910–918
I Guideline (2018) Validation of analytical procedures: text and methodology, Q2 (2005) http://somatekcom/content/uploads/2014/06/sk140605hpdf, February 28
Salvador A, Millerioux L, Renou A (2006) Simultaneous LC-MS-MS analysis of capecitabine and its metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil) after off-line SPE from human plasma. Chromatographia 63:609–615
Montange D, Berard M, Demarchi M, Muret P, Piédoux S, Kantelip JP (2010) An APCI LC-MS/MS method for routine determination of capecitabine and its metabolites in human plasma. J Mass Spectrom 45:670–677
Baymak MS, Celik H, Ozkan SA (2015) The application of differential pulse polarography to the analysis of capecitabine and investigation of its Electroreduction mechanism. J Electrochem Soc 162:G29–G35
Mader RM, Schrolnberger C, Rizovski B, Brunner M, Wenzel C, Locker G (2003) Penetration of capecitabine and its metabolites into malignant and healthy tissues of patients with advanced breast cancer. Br J Cancer 88:782–787
Farkouh A, Ettlinger D, Schueller J, Georgopoulos A, Scheithauer W, Czejka M (2010) A rapid and simple HPLC assay for quantification of capecitabine for drug monitoring purposes. Anticancer Res 30:5207–5211
Švobaitė R, Solassol I, Pinguet F, Mazard T, Ivanauskas L, Ychou M (2010) A liquid chromatography-mass spectrometry method for the simultaneous determination of capecitabine, 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil, and 5-fluorodihydrouracil in human plasma. J Liq Chromatogr Relat Technol 33:1705–1719
Singhal P, Shah PA, Shah JV, Sharma P, Shrivastav PS (2015) Determination of capecitabine-An anti-cancer drug in dried blood spot by LC–ESI–MS/MS. Int J Pharm Pharm Sci 7:238–245
Funding
The authors are grateful to the University of Kashan for supporting this work by Grant No. 890412/1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committee of the public health of Iranian blood transfusion service and was performed in accordance with the ethical standards. Informed consent was obtained from individual participant included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4042 kb)
Rights and permissions
About this article
Cite this article
Ansari, S., Masoum, S. Ultrasound-assisted dispersive solid-phase microextraction of capecitabine by multi-stimuli responsive molecularly imprinted polymer modified with chitosan nanoparticles followed by HPLC analysis. Microchim Acta 187, 366 (2020). https://doi.org/10.1007/s00604-020-04345-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04345-0