Abstract
In this study molecularly imprinted polymers (MIP) based on carbon quantum dots (CQDs) and polymer dots (PDs) are developed for selective determination of acetamiprid using fluorometry. The measurement is based on the fluorescence quenching of CQDs and PDs in the presence of acetamiprid. PDs were prepared using a one-step aqueous synthesis method from ascorbic acid and diethylenetriamine at room temperature. CQDs were prepared from the same materials using the hydrothermal method at 180 °C. These particles were characterized using field emission scanning electron microscopy (FE-SEM), FTIR, dynamic light scattering (DLS), X-ray diffraction (XRD), UV–Vis, and fluorescence. The quantum yield was 47% for PDs and 8% for CQDs. Then, molecularly imprinted polymers (MIP) were prepared based on PDs and CQDs using reverse microemulsion method. The fluorescence quenching of CQD@MIPs and PD@MIPs was investigated at an excitation wavelength of 350 nm and emission wavelength of 440 nm in the presence of a template. Other variables affecting the fluorescence peaking were optimized using design expert software. The results illustrate that the use of PD@MIPs had a wide dynamic range 0.08–109 nmol L−1, good accuracy and detection limit of 0.02 nmol L−1, while using CQD@MIPs led to a lower dynamic range 0.36–64 nmol L−1, and detection limit of only 0.11 nmol L−1. The responses of the optical nanoprobe for acetamiprid in water (recovery 92–102%) and apple (recovery 92–103%) were also investigated.

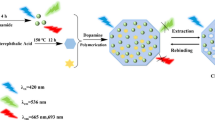
Schematic representation of preparation polymer dots (PDs), carbon quantum dots (CQDs), PDs coated with imprinted polymers (PD@MIPs), and CQDs coated with imprinted polymers (CQD@MIPs) in the presence and absence of acetamiprid



Similar content being viewed by others
References
Verdian A (2018) Apta-nanosensors for detection and quantitative determination of acetamiprid–a pesticide residue in food and environment. Talanta 176:456–464. https://doi.org/10.1016/j.talanta.2017.08.070
Pesticide residues in food 2011. Jt FAO/WHO Meet Pestic Residues FAO PLANT:23
Tian Y, Wang Y, Sheng Z, Li T, Li X (2016) A colorimetric detection method of pesticide acetamiprid by fine-tuning aptamer length. Anal Biochem 513:87–92. https://doi.org/10.1016/j.ab.2016.09.004
Mao Y, Bao Y, Han D, Li F, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosens Bioelectron 38:55–60. https://doi.org/10.1016/j.bios.2012.04.043
Yang L, Sun H, Wang X, Yao W, Zhang W, Jiang L (2019) An aptamer based aggregation assay for the neonicotinoid insecticide acetamiprid using fluorescent upconversion nanoparticles and DNA functionalized gold nanoparticles. Microchim Acta 186:308. https://doi.org/10.1007/s00604-019-3422-9
Saberi Z, Rezaei B, Ensafi AA (2019) Fluorometric label-free aptasensor for detection of the pesticide acetamiprid by using cationic carbon dots prepared with cetrimonium bromide. Microchim Acta 186:273. https://doi.org/10.1007/s00604-019-3378-9
Bahreyni A, Yazdian-Robati R, Ramezani M, Abnous K, Taghdisi SM (2018) Fluorometric aptasensing of the neonicotinoid insecticide acetamiprid by using multiple complementary strands and gold nanoparticles. Microchim Acta 185:272. https://doi.org/10.1007/s00604-018-2805-7
Wu C, Bull B, Szymanski C, Christensen K, McNeill J (2008) Multicolor conjugated polymer dots for biological fluorescence imaging. ACS Nano 2:2415–2423. https://doi.org/10.1021/nn800590n
Sun J, Mei H, Gao F (2017) Ratiometric detection of copper ions and alkaline phosphatase activity based on semiconducting polymer dots assembled with rhodamine B hydrazide. Biosens Bioelectron 91:70–75. https://doi.org/10.1016/j.bios.2016.12.034
Ke CS, Fang CC, Yan JY, Tseng PJ, Pyle JR, Chen CP, Lin SY, Chen J, Zhang X, Chan YH (2017) Molecular engineering and design of semiconducting polymer dots with narrow-band, near-infrared emission for in vivo biological imaging. ACS Nano 11:3166–3177. https://doi.org/10.1021/acsnano.7b00215
Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8:355–381. https://doi.org/10.1007/s12274-014-0644-3
Sun K, Chen H, Wang L, Yin S, Wang H, Xu G, Chen D, Zhang X, Wu C, Qin W (2014) Size-dependent property and cell labeling of semiconducting polymer dots. ACS Appl Mater Interfaces 6:10802–10812. https://doi.org/10.1021/am502733n
Xia J, Zhuang YT, Yu YL, Wang JH (2017) Highly fluorescent carbon polymer dots prepared at room temperature, and their application as a fluorescent probe for determination and intracellular imaging of ferric ion. Microchim Acta 184:1109–1116. https://doi.org/10.1007/s00604-017-2104-8
Ensafi AA, Kazemifard N, Rezaei B (2017) Development of a selective prilocaine optical sensor based on molecularly imprinted shell on CdTe quantum dots. Sensors Actuators B Chem 242:835–841. https://doi.org/10.1016/j.snb.2016.09.175
Guo Z, Gai L, Zhou J, Jiang H, Tian Y (2016) Polybromopyrrole-derived nitrogen-containing polymer dots: synthesis, optical properties, and insight into their fluorescence quenching by aromatic compounds. Sensors Actuators B Chem 232:722–731. https://doi.org/10.1016/j.snb.2016.04.047
Yang CZ, Liu YC, Xu C, Bai AM, Hu YJ (2020) A sensitive fluorescent sensor based on the photoinduced electron transfer mechanism for cefixime and ctDNA. J Mol Recognit 33:e2816. https://doi.org/10.1002/jmr.2816
Ding H, Jiao HF, Shi XZ, Sun AL, Guo XQ, Li DX, Chen J (2017) Molecularly imprinted optosensing sensor for highly selective and sensitive recognition of sulfadiazine in seawater and shrimp samples. Sensors Actuators B Chem 246:510–517. https://doi.org/10.1016/j.snb.2017.02.096
Li X, Jiao HF, Shi XZ, Sun A, Wang X, Chai J, Li DX, Chen J (2018) Development and application of a novel fluorescent nanosensor based on FeSe quantum dots embedded silica molecularly imprinted polymer for the rapid optosensing of cyfluthrin. Biosens Bioelectron 99:268–273. https://doi.org/10.1016/j.bios.2017.07.071
Liu H, Zhou K, Wu D, Wang J, Sun B (2016) A novel quantum dots-labeled on the surface of molecularly imprinted polymer for turn-off optosensing of dicyandiamide in dairy products. Biosens Bioelectron 77:512–517. https://doi.org/10.1016/j.bios.2015.10.007
Liu X, Li Y, Liang J, Zhu W, Xu J, Su R, Yuan L, Sun C (2016) Aptamer contained triple-helix molecular switch for rapid fluorescent sensing of acetamiprid. Talanta 160:99–105. https://doi.org/10.1016/j.talanta.2016.07.010
Hu J, Ni P, Dai H, Sun Y, Wang Y, Jiang S, Li Z (2015) A facile label-free colorimetric aptasensor for ricin based on the peroxidase-like activity of gold nanoparticles. RSC Adv 5:16036–16041. https://doi.org/10.1039/c4ra17327a
Sun N, Ding Y, Tao Z, You H, Hua X, Wang M (2018) Development of an upconversion fluorescence DNA probe for the detection of acetamiprid by magnetic nanoparticles separation. Food Chem 257:289–294. https://doi.org/10.1016/j.foodchem.2018.02.148
Lin B, Yu Y, Li R, Cao Y, Guo M (2016) Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sensors Actuators B Chem 229:100–109. https://doi.org/10.1016/j.snb.2016.01.114
Xiang L, Tang J (2017) QD-aptamer as a donor for a FRET-based chemosensor and evaluation of affinity between acetamiprid and its aptamer. RSC Adv 7:8332–8337. https://doi.org/10.1039/c6ra26118c
Hu W, Chen Q, Li H, Ouyang Q, Zhao J (2016) Erratum: corrigendum to “Fabricating a novel label-free aptasensor for acetamiprid by fluorescence resonance energy transfer between NH 2 -NaYF 4 : Yb, ho@SiO 2 and Au nanoparticles” [Biosens. Bioelectron. (2016) 80:398–404] Biosensors and Bioelectronics. Biosens Bioelectron 85:997. https://doi.org/10.1016/j.bios.2016.05.012
Abnous K, Danesh NM, Ramezani M, Alibolandi M, Lavaee P, Taghdisi SM (2017) Aptamer based fluorometric acetamiprid assay using three kinds of nanoparticles for powerful signal amplification. Microchim Acta 184:81–90. https://doi.org/10.1007/s00604-016-1992-3
Guo J, Li Y, Wang L, Xu J, Huang Y, Luo Y, Shen F, Sun C, Meng R (2016) Aptamer-based fluorescent screening assay for acetamiprid via inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Anal Bioanal Chem 408:557–566. https://doi.org/10.1007/s00216-015-9132-1
Qi Y, Xiu FR, Zheng M, Li B (2016) A simple and rapid chemiluminescence aptasensor for acetamiprid in contaminated samples: sensitivity, selectivity and mechanism. Biosens Bioelectron 83:243–249. https://doi.org/10.1016/j.bios.2016.04.074
Wang C, Chen D, Wang Q, Wang Q (2016) Aptamer-based resonance light scattering for sensitive detection of acetamiprid. Anal Sci 32:757–762. https://doi.org/10.2116/analsci.32.757
Fan L, Zhao G, Shi H, Liu M, Li Z (2013) A highly selective electrochemical impedance spectroscopy-based aptasensor for sensitive detection of acetamiprid. Biosens Bioelectron 43:12–18. https://doi.org/10.1016/j.bios.2012.11.033
Rapini R, Cincinelli A, Marrazza G (2016) Acetamiprid multidetection by disposable electrochemical DNA aptasensor. Talanta 161:15–21. https://doi.org/10.1016/j.talanta.2016.08.026
Taghdisi SM, Danesh NM, Ramezani M, Abnous K (2017) Electrochemical aptamer based assay for the neonicotinoid insecticide acetamiprid based on the use of an unmodified gold electrode. Microchim Acta 184:499–505. https://doi.org/10.1007/s00604-016-2038-6
Li H, Qiao Y, Li J, Fang H, Fan D, Wang W (2016) A sensitive and label-free photoelectrochemical aptasensor using Co-doped ZnO diluted magnetic semiconductor nanoparticles. Biosens Bioelectron 77:378–384. https://doi.org/10.1016/j.bios.2015.09.066
Acknowledgments
We wish to thank Isfahan University of Technology (IUT) Research Council and Center of Excellence in Sensor and Green Chemistry for their patronage and help in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1686 kb)
Rights and permissions
About this article
Cite this article
Ghani, S.M., Rezaei, B., Jamei, H.R. et al. Preparation and comparison of molecularly imprinted polymer fluorimetric nanoprobe based on polymer dots and carbon quantum dots for determination of acetamiprid using response surface method. Microchim Acta 187, 294 (2020). https://doi.org/10.1007/s00604-020-04283-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04283-x