Abstract
An aptasensor is described for the ultrasensitive detection of Pseudomonas aeruginosa (P. aeruginosa). A glassy carbon electrode (GCE) was first modified by electrodeposition of silver nanoparticles (AgNPs). Immobilization of NH2-aptamer was covalently attached to the AgNP/GCE surface. The morphology, distribution and size of the sensor were characterized by field emission scanning electron microscopy. Cyclic voltammetry and electrical impedance spectroscopy were used to study conductivity of the aptasensor and the electrochemical properties. Detection of P. aeruginosa was carried out by evaluation of the charge transfer resistance after and before the adding of P.aeruginosa and by using the hexacyanoferrate redox system as an electrochemical probe. The impedance increases on going from 102 to 107 CFU·mL−1 concentrations of P. aeruginosa, and the detection limit is 33 CFU·mL−1 (for S/N = 3). The assay was successfully applied for the determination of P. aeruginosa in spiker serum samples.

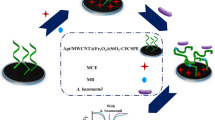
Schematic representation of an impedimetric assay for Pseudomonas aeruginosa by using a [Fe(CN)6]3−/4- probe based on immobilization of amino-modified aptamer onto a glassy carbon electrode (GCE) modified with silver nanoparticles (AgNPs): Incubation with P. aeruginosa leads to an increase of the charge-transfer resistance.






Similar content being viewed by others
References
Krithiga N, Viswanath KB, Vasantha VS, Jayachitra A (2016) Specific and selective electrochemical immunoassay for Pseudomonas aeruginosa based on pectin–gold nano composite. Biosens Bioelectron 79:121–129
Sismaet HJ, Pinto AJ, Goluch ED (2017) Electrochemical sensors for identifying pyocyanin production in clinical Pseudomonas aeruginosa isolates. Biosens Bioelectron 97:65–69
Wiehlmann L, Cramer N, Ulrich J et al (2012) Effective prevention of Pseudomonas aeruginosa cross-infection at a cystic fibrosis centre–results of a 10-year prospective study. Int J Med Microbiol 302:69–77
Kingsford NM, Raadsma HW (1995) Detection of Pseudomonas aeruginosa from ovine fleece washings by PCR amplification of 16S ribosomal RNA. Vet Microbiol 47:61–70
Kodaka H, Iwata M, Yumoto S, Kashitani F (2003) Evaluation of a new agar medium containing cetrimide, kanamycin and nalidixic acid for isolation and enhancement of pigment production of Pseudomonas aeruginosa in clinical samples. J Basic Microbiol An Int J Biochem Physiol Genet Morphol Ecol Microorg 43:407–413
Shibuya N, Kohno S, Shigeno Y et al (1987) Rapid diagnosis of respiratory pseudomonas infections--indirect immunofluorescent-staining of sputum with monoclonal antibody to Pseudomonas aeruginosa. Kansenshogaku Zasshi 61:1406
Xu Y, Theobald V, Sung C et al (2008) Validation of a HLA-A2 tetramer flow cytometric method, IFNgamma real time RT-PCR, and IFNgamma ELISPOT for detection of immunologic response to gp100 and MelanA/MART-1 in melanoma patients. J Transl Med 6:61
Imperi F, Ciccosanti F, Perdomo AB et al (2009) Analysis of the periplasmic proteome of Pseudomonas aeruginosa, a metabolically versatile opportunistic pathogen. Proteomics 9:1901–1915
Webster TA, Sismaet HJ, Conte JL, Goluch ED (2014) Electrochemical detection of Pseudomonas aeruginosa in human fluid samples via pyocyanin. Biosens Bioelectron 60:265–270
Li F, Zhang H, Wang Z et al (2014) Aptamers facilitating amplified detection of biomolecules. Anal Chem 87:274–292
Labib M, Zamay AS, Kolovskaya OS et al (2012) Aptamer-based impedimetric sensor for bacterial typing. Anal Chem 84:8114–8117
Valencia-Burton M, Broude NE (2007) Visualization of RNA Using Fluorescence Complementation Triggered by Aptamer-Protein Interactions (RFAP) in Live Bacterial Cells. Curr Protoc cell Biol 37:11–17
Yang C, Wang Q, Xiang Y, Yuan R, Chai Y (2014) Target-induced strand release and thionine-decorated gold nanoparticle amplification labels for sensitive electrochemical aptamer-based sensing of small molecules. Sensors Actuators B Chem 197:149–154
Yang M, Javadi A, Li H, Gong S (2010) Ultrasensitive immunosensor for the detection of cancer biomarker based on graphene sheet. Biosens Bioelectron 26:560–565
Zhang H, Li X, Chen G (2009) Ionic liquid-facilitated synthesis and catalytic activity of highly dispersed Ag nanoclusters supported on TiO2. J Mater Chem 19:8223–8231
Salimi A, Kavosi B, Fathi F, Hallaj R (2013) Highly sensitive immunosensing of prostate-specific antigen based on ionic liquid–carbon nanotubes electrode: application as cancer biomarker for prostatebiopsies. Biosens Bioelectron 42:439–446
Shahdost-fard F, Salimi A, Sharifi E, Korani A (2013) Fabrication of a highly sensitive adenosine aptasensor based on covalent attachment of aptamer onto chitosan–carbon nanotubes–ionic liquid nanocomposite. Biosens Bioelectron 48:100–107
Yu L, Shi Y, Zhao Z, Yin H, Wei Y, Liu J, Kang W, Jiang T, Wang A (2011) Ultrasmall silver nanoparticles supported on silica and their catalytic performances for carbon monoxide oxidation. Catal Commun 12:616–620
Roushani M, Shahdost-fard F (2015) A novel ultrasensitive aptasensor based on silver nanoparticles measured via enhanced voltammetric response of electrochemical reduction of riboflavin as redox probe for cocaine detection. Sensors Actuators B Chem 207:764–771
Zanella R, Giorgio S, Shin CH, Henry CR, Louis C (2004) Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J Catal 222:357–367 16
Moreau F, Bond GC, Taylor AO (2005) Gold on titania catalysts for the oxidation of carbon monoxide: control of pH during preparation with various gold contents. J Catal 231:105–114
Roushani M, Shahdost-fard F (2018) A glassy carbon electrode with electrodeposited silver nanoparticles for aptamer based voltammetric determination of trinitrotoluene using riboflavin as a redox probe. Microchim Acta 185:558
Zhong Z, Gao X, Gao R, Jia L (2018) Selective capture and sensitive fluorometric determination of Pseudomonas aeruginosa by using aptamer modified magnetic nanoparticles. Microchim Acta 185(8):377
Labib M, Zamay AS, Kolovskaya OS, Reshetneva IT, Zamay GS, Kibbee RJ, Sattar SA, Zamay TN, Berezovski MV (2012) Aptamer-based viability impedimetric sensor for bacteria. Anal Chem 84:8966–8969
Cai W-Y, Xu Q, Zhao X-N et al (2006) Porous gold-Nanoparticle− CaCO3 hybrid material: preparation, characterization, and application for horseradish Peroxidase assembly and direct electrochemistry. Chem Mater 18:279–284
Baldock BL, Hutchison JE (2016) UV–Visible Spectroscopy-Based Quantification of Unlabeled DNA Bound to Gold Nanoparticles. Anal Chem 88:12072–12080
Shahdost-fard F, Roushani M (2016) An impedimetric aptasensor based on water soluble cadmium telluride (CdTe) quantum dots (QDs) for detection of ibuprofen. J Electroanal Chem 763:18–24
Wu Z, He D, Cui B, Jin Z (2018) A bimodal (SERS and colorimetric) aptasensor for the detection of Pseudomonas aeruginosa. Microchim Acta 11:185–528
Jia F, Xu L, Yan W, Wu W, Yu Q, Tian X, Dai R, Li X (2017) A magnetic relaxation switch aptasensor for the rapid detection of Pseudomonas aeruginosa using superparamagnetic nanoparticles. Microchim Acta 184:1539–1545
Kaur G, Raj T, Kaur N, Singh N (2015) Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa. Org Biomol Chem 13:4673–4679
Acknowledgements
This study has been supported by the Ilam University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 66.3 kb)
Rights and permissions
About this article
Cite this article
Roushani, M., Sarabaegi, M. & Pourahmad, F. Impedimetric aptasensor for Pseudomonas aeruginosa by using a glassy carbon electrode modified with silver nanoparticles. Microchim Acta 186, 725 (2019). https://doi.org/10.1007/s00604-019-3858-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3858-y