Abstract
An electrochemical sensor is described for simultaneous determination of hydroquinone (HQ) and catechol (CT) via differential pulse voltammetry (DPV). It is making use of a ternary composite material prepared from oxidized multiwalled carbon nanotubes, manganese dioxide (MnO2) and manganese ferrite (MnFe2O4). The material was obtained by a one-step hydrothermal reaction and used to modify a glassy carbon electrode (GCE). The composite was characterized by Fourier transform infrared spectroscopy, X-ray powder diffraction, thermogravimetric analysis, X-ray photoelectron spectroscopy and scanning electron microscopy. The peak currents for HQ and CT are highest at 172 and 276 mV (vs. Ag/AgCl) at a pH value of 6.0. Response increases linearly in the 1–400 μM HQ and CT concentration ranges, and the detection limits are 0.64 and 0.48 μM, respectively. The modified GCE is highly selective, repeatable and reproducible. A single sensor was used to make 23 subsequent measurements, and the relative standard deviations were 1.8% and 2.3% for HQ and CT, respectively.

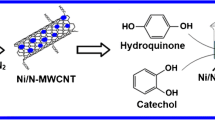
Schematic representation of the preparation of ternary nanocomposite and its electrochemical behavior towards hydroquinone and catechol.





Similar content being viewed by others
References
Chen Y, Liu X, Zhang S, Yang L, Liu M, Zhang Y, Yao S (2017) Ultrasensitive and simultaneous detection of hydroquinone, catechol and resorcinol based on the electrochemical co-reduction prepared Au-Pd nanoflower/reduced graphene oxide nanocomposite. Electrochim Acta 231:677–685
Xie T, Liu Q, Shi Y, Liu Q (2006) Simultaneous determination of positional isomers of benzenediols by capillary zone electrophoresis with square wave amperometric detection. J Chromatogr A 1109:317–321
Fragoso S, Acena L, Guasch J, Mestres M, Busto O (2011) Quantification of phenolic compounds during red winemaking using FT-MIR spectroscopy and PLS-regression. J Agric Food Chem 59:10795–10802
Guan N, Zeng Z, Wang Y, Fu E, Cheng J (2000) Open tubular capillary electrochromatography in fused-silica capillaries chemically bonded with macrocyclic dioxopolyamine. Anal Chim Acta 4180:145–151
He JF, Yao FJ, Cui H, Li XJ, Yuan ZB (2012) Simultaneous determination of dihydroxybenzene positional isomers by capillary electrochromatography using gold nanoparticles as stationary phase. J Sep Sci 35:1003–1009
Wang Y, Wu Y, Xie J, Ge H, Hu X (2013) Multi-walled carbon nanotubes and metal-organic framework nanocomposites as novel hybrid electrode materials for the determination of nano-molar levels of lead in a lab-on-valve format. Analyst 138:5113–5120
Guzsvány V, Vajdle O, Gurdeljević M, Kónya Z (2018) Ag or Au nanoparticles decorated multiwalled carbon nanotubes coated carbon paste electrodes for Amperometric determination of H2O2. Top Catal 61:1350–1361
Li C, Wu Z, Yang H, Deng L, Chen X (2017) Reduced graphene oxide-cyclodextrin-chitosan electrochemical sensor: effective and simultaneous determination of o- and p-nitrophenols. Sensor Actuat B-Chem 251:446–454
Fotouhi L, Dorraji PS, Keshmiri YSS, Hamtak M (2018) Electrochemical sensor based on nanocomposite of multi-walled carbon nanotubes / TiO2Nanoparticles in chitosan matrix for simultaneous and separate determination of Dihydroxybenzene isomers. J Electrochem Soc 165:B202–B211
Balram D, Lian K-Y, Sebastian N (2018) Synthesis of a functionalized multi-walled carbon nanotube decorated ruskin michelle-like ZnO nanocomposite and its application in the development of a highly sensitive hydroquinone sensor. Inorg Chem Front 5:1950–1961
Tang J, Jin B (2015) A voltammetric sensor based on multi-walled carbon nanotubes-MnO2 nanowires composite film for simultaneous determination of hydroquinone and catechol. Anal Methods-UK 7:9218–9225
Zhang H, Wu L (2018) Na+ intercalated manganese dioxide/MOF-derived Nanoporous carbon hybrid electrodes for supercapacitors with high rate performance and cyclic stability. J Electrochem Soc 165:2815–2823
Ladrak T, Smulders S, Roubeau O, Teat SJ, Gamez P, Reedijk J (2010) Manganese-based metal-organic frameworks as heterogeneous catalysts for the Cyanosilylation of acetaldehyde. Eur J Inorg Chem 2010:3804–3812
Luna-Lama F, Hernández-Rentero C, Caballero A, Morales J (2018) Biomass-derived carbon/γ-MnO2 nanorods/S composites prepared by facile procedures with improved performance for Li/S batteries. Electrochim Acta 292:522–531
Ravindran Madhura T, Viswanathan P, Gnana kumar G, Ramaraj R (2017) Nanosheet-like manganese ferrite grown on reduced graphene oxide for non-enzymatic electrochemical sensing of hydrogen peroxide. J Electroanal Chem 792:15–22
Kafshgari LA, Ghorbani M, Azizi A, Agarwal S, Gupta VK (2017) Modeling and optimization of direct red 16 adsorption from aqueous solutions using nanocomposite of MnFe2O4 /MWCNTs: RSM-CCRD model. J Mol Liq 233:370–377
Singh G, Chandra S (2018) Electrochemical performance of MnFe2O4 nano-ferrites synthesized using thermal decomposition method. Int J Hydrogen Energ 43:4058–4066
Zha D, Xiong P, Wang X (2015) Strongly coupled manganese ferrite/carbon black/polyaniline hybrid for low-cost supercapacitors with high rate capability. Electrochim Acta 185:218–228
Liang C, Feng X, Yu J, Jiang X (2018) Facile one-step hydrothermal syntheses of graphene oxide–MnO2 composite and their application in removing heavy metal ions. Micro Nano Lett 13:1179–1184
Luo J, Hu C, Meng X, Crittenden J, Qu J, Peng P (2017) Antimony removal from aqueous solution using novel α-MnO2 nanofibers: equilibrium, kinetic, and density functional theory studies. ACS Sustain Chem Eng 5:2255–2264
Wu K, Hu G, Cao Y, Peng Z, Du K (2015) Facile and green synthesis of MnFe2O4/reduced graphene oxide nanocomposite as anode materials for Li-ion batteries. Mater Lett 161:178–180
Barai HR, Banerjee AN, Bai F, Joo SW (2018) Surface modification of titania nanotube arrays with crystalline manganese-oxide nanostructures and fabrication of hybrid electrochemical electrode for high-performance supercapacitors. J Ind Eng Chem 62:409–417
Xiong P, Hu C, Fan Y, Zhang W, Zhu J, Wang X (2014) Ternary manganese ferrite/graphene/polyaniline nanostructure with enhanced electrochemical capacitance performance. J Power Sources 266:384–392
Peng Y, Tang Z, Dong Y, Che G, Xin Z (2018) Electrochemical detection of hydroquinone based on MoS2 /reduced graphene oxide nanocomposites. J Electroanal Chem 816:38–44
Huang H, Zhang J, Cheng M, Liu K, Wang X (2017) Amperometric sensing of hydroquinone using a glassy carbon electrode modified with a composite consisting of graphene and molybdenum disulfide. Microchim Acta 184(12):4803–4808
Xu J, Xia J, Zhang F, Wang Z (2017) An electrochemical sensor based on metal-organic framework-derived porous carbon with high degree of graphitization for electroanalysis of various substances. Electrochim Acta 251:71–80
Jian X, Liu X, Yang H-M, Guo M-M, Song X-L, Dai H-Y, Liang Z-H (2016) Graphene quantum dots modified glassy carbon electrode via electrostatic self-assembly strategy and its application. Electrochim Acta 190:455–462
Tashkhourian J, Daneshi M, Nami-Ana F, Behbahani M, Bagheri A (2016) Simultaneous determination of hydroquinone and catechol at gold nanoparticles mesoporous silica modified carbon paste electrode. J Hazard Mater 318:117–124
Kuskur CM, Kumara Swamy BE, Jayadevappa H (2017) Poly (naphthol green B) modified carbon paste electrode sensor for catechol and hydroquinone. J Electroanal Chem 804:99–106
Si W, Lei W, Zhang Y, Xia M, Wang F, Hao Q (2012) Electrodeposition of graphene oxide doped poly(3,4-ethylenedioxythiophene) film and its electrochemical sensing of catechol and hydroquinone. Electrochim Acta 85:295–301
Feng S, Zhang Y, Zhong Y, Li Y, Li S (2014) Simultaneous determination of hydroquinone and catechol using covalent layer-by-layer self-assembly of carboxylated-MWNTs. J Electroanal Chem 733:1–5
Xiang Y, li L, liu H, Shi Z, Y Tan CW, Liu Y, Wang J, Zhang S (2018) One-step synthesis of three-dimensional interconnected porous carbon and their modified electrode for simultaneous determination of hydroquinone and catechol. Sensor Actuat B-Chem 267:302–311
Wu Y, Lei W, Xia M, Wang F, Li C, Zhang C, Hao Q, Zhang Y (2018) Simultaneous electrochemical sensing of hydroquinone and catechol using nanocomposite based on palygorskite and nitrogen doped graphene. Appl Clay Sci 162:38–45
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21571191 and No. 51674292) and Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety (2018TP1003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 885 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Huang, R., Yu, J. et al. Simultaneous voltammetric determination of hydroquinone and catechol by using a glassy carbon electrode modified with a ternary nanocomposite prepared from oxidized multiwalled carbon nanotubes, manganese dioxide and manganese ferrite. Microchim Acta 186, 643 (2019). https://doi.org/10.1007/s00604-019-3750-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3750-9