Abstract
A rapid lateral flow immunoassay is presented that uses carboxyl-modified superparamagnetic nanoparticles as labels that can be quantified by highly sensitive multi-channel electronic readers. The approach is generic in that it is likely to be applicable to numerous small molecules. The method permits both single- and multiplex assays at a point-of-need without sample pretreatment. It is user-friendly and offers attractive characteristics demonstrated here for detection of morphine, fentanyl and methamphetamine in urine. The competitive immunoassay uses commercially available reagents that do not require special permissions. After migration of sample, the lateral flow test strips are subjected to an alternating magnetic field at two frequencies. The response from the nanolabels is readout at a combinatorial frequency from the entire volume of a porous immunochromatographic membrane by the magnetic particle quantification technique. Even trace concentrations can be quantified within ≤20 min with the limits of detection (LOD) of 0.20 ng·mL−1, 0.36 ng·mL−1 and 1.30 ng·mL−1 for morphine, fentanyl and methamphetamine, respectively. The second variant presented here features highly sensitive quantification of haptens (LOD for fentanyl - 0.05 ng·mL−1). This is due to high-affinity trapping of magnetic nanolabels in a universal streptavidin-based test strip, which can be also used for detection of virtually any other small molecule. The third variant is of the multiplexed type and intended for rapid and simultaneous detection of the drugs of abuse in human urine with LODs equal to 0.60 ng·mL−1 and 3.0 ng·mL−1 for morphine and methamphetamine, respectively. In addition to the low LODs, the RSDs did not exceed 7%, 9%, and 11% for methamphetamine, morphine and fentanyl, respectively.

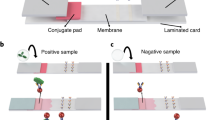
Three variants of small molecule detection in competitive format at a point-of-need. Single-plex variants feature antibody and high-affinity streptavidin test lines, while multiplex variant - several antibody test lines. Magnetic nanolabels are quantified from the whole volume of test strip.






Similar content being viewed by others
References
Evans AM, DeHaven CD, Barrett T et al (2009) Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81:6656–6667. https://doi.org/10.1021/ac901536h
Cappiello A, Famiglini G, Palma P, Pierini E, Termopoli V, Trufelli H (2011) Direct-EI in LC-MS: towards a universal detector for small-molecule applications. Mass Spectrom Rev 30:1242–1255. https://doi.org/10.1002/mas.20329
Ivanov AE, Pushkarev AV, Orlov AV, Nikitin MP, Nikitin PI (2019) Interferometric detection of chloramphenicol via its immunochemical recognition at polymer-coated nano-corrugated surfaces. Sensors Actuators B Chem 282:984–991. https://doi.org/10.1016/j.snb.2018.11.043
Mauriz E, Calle A, Abad A, Montoya A, Hildebrandt A, Barceló D, Lechuga LM (2006) Determination of carbaryl in natural water samples by a surface plasmon resonance flow-through immunosensor. Biosens Bioelectron 21:2129–2136. https://doi.org/10.1016/j.bios.2005.10.013
Sang S, Wang Y, Feng Q, Wei Y, Ji J, Zhang W (2016) Progress of new label-free techniques for biosensors: a review. Crit Rev Biotechnol 36:465–481. https://doi.org/10.3109/07388551.2014.991270
Orlov AV, Pushkarev AV, Mochalova EN, Nikitin PI, Nikitin MP (2018) Development and label-free investigation of logic-gating biolayers for smart biosensing. Sensors Actuators B Chem 257:971–979. https://doi.org/10.1016/j.snb.2017.11.025
Yu S, Wei Q, Du B et al (2013) Label-free immunosensor for the detection of kanamycin using ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens Bioelectron 48:224–229. https://doi.org/10.1016/j.bios.2013.04.025
Shevchenko KG, Cherkasov VR, Tregubov AA, Nikitin PI, Nikitin MP (2017) Surface plasmon resonance as a tool for investigation of non-covalent nanoparticle interactions in heterogeneous self-assembly & disassembly systems. Biosens Bioelectron 88:3–8. https://doi.org/10.1016/j.bios.2016.09.042
Smith DS, Eremin SA (2008) Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal Bioanal Chem 391:1499–1507. https://doi.org/10.1007/s00216-008-1897-z
Farka Z, Juřík T, Kovář D, Trnková L, Skládal P (2017) Nanoparticle-based immunochemical biosensors and assays: recent advances and challenges. Chem Rev 117:9973–10042. https://doi.org/10.1021/acs.chemrev.7b00037
Fodey T, Leonard P, O’Mahony J, O’Kennedy R, Danaher M (2011) Developments in the production of biological and synthetic binders for immunoassay and sensor-based detection of small molecules. TrAC - Trends Anal Chem 30:254–269. https://doi.org/10.1016/j.trac.2010.10.011
Goryacheva IY, Lenain P, De Saeger S (2013) Nanosized labels for rapid immunotests. TrAC Trends Anal Chem 46:30–43. https://doi.org/10.1016/j.trac.2013.01.013
Tregubov AA, Nikitin PI, Nikitin MP (2018) Advanced smart nanomaterials with integrated logic-gating and Biocomputing: Dawn of Theranostic Nanorobots. Chem Rev 118:10294–10348. https://doi.org/10.1021/acs.chemrev.8b00198
Weller MG (2000) Immunochromatographic techniques - a critical review. Fresenius J Anal Chem 366:635–645. https://doi.org/10.1007/s002160051558
Teerinen T, Lappalainen T, Erho T (2014) A paper-based lateral flow assay for morphine. Anal Bioanal Chem 406:5955–5965. https://doi.org/10.1007/s00216-014-8001-7
Sajid M, Kawde AN, Daud M (2015) Designs, formats and applications of lateral flow assay: a literature review. J Saudi Chem Soc 19:689–705. https://doi.org/10.1016/j.jscs.2014.09.001
Taranova NA, Byzova NA, Zaiko VV, Starovoitova TA, Vengerov YY, Zherdev AV, Dzantiev BB (2013) Integration of lateral flow and microarray technologies for multiplex immunoassay: application to the determination of drugs of abuse. Microchim Acta 180:1165–1172. https://doi.org/10.1007/s00604-013-1043-2
Lee JR, Choi J, Shultz TO, Wang SX (2016) Small molecule detection in saliva facilitates portable tests of marijuana abuse. Anal Chem 88:7457–7461. https://doi.org/10.1021/acs.analchem.6b01688
Znoyko SL, Orlov AV, Pushkarev AV, Mochalova EN, Guteneva NV, Lunin AV, Nikitin MP, Nikitin PI (2018) Ultrasensitive quantitative detection of small molecules with rapid lateral-flow assay based on high-affinity bifunctional ligand and magnetic nanolabels. Anal Chim Acta 1034:161–167. https://doi.org/10.1016/j.aca.2018.07.012
Navaee A, Salimi A, Teymourian H (2012) Graphene nanosheets modified glassy carbon electrode for simultaneous detection of heroine, morphine and noscapine. Biosens Bioelectron 31:205–211. https://doi.org/10.1016/j.bios.2011.10.018
Nikitin PI, Vetoshko PM, Ksenevich TI (2007) New type of biosensor based on magnetic nanoparticle detection. J Magn Magn Mater 311:445–449. https://doi.org/10.1016/j.jmmm.2006.10.1180
Orlov AV, Znoyko SL, Cherkasov VR, Nikitin MP, Nikitin PI (2016) Multiplex biosensing based on highly sensitive magnetic Nanolabel quantification: rapid detection of botulinum neurotoxins a, B, and E in liquids. Anal Chem 88:10419–10426. https://doi.org/10.1021/acs.analchem.6b02066
National Institute on Drug Abuse (NIDA), Overdose Death Rates, Revised January 2019: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed 9 Apr 2019
Winek CL, Wahba WW, Winek CL, Balzer TW (2001) Drug and chemical blood-level data 2001. Forensic Sci Int 122:107–123. https://doi.org/10.1016/S0379-0738(01)00483-2
Hermanson GT (2013) Bioconjugate techniques: third edition. Academic Press, NewYork. https://doi.org/10.1016/C2009-0-64240-9
Ye H, Xia X (2018) Enhancing the sensitivity of colorimetric lateral flow assay (CLFA) through signal amplification techniques. J Mater Chem B 6:7102–7111. https://doi.org/10.1039/C8TB01603H
Shipunova VO, Nikitin MP, Nikitin PI, Deyev SM (2016) MPQ-cytometry: a magnetism-based method for quantification of nanoparticle–cell interactions. Nanoscale 8:12764–12772. https://doi.org/10.1039/C6NR03507H
Orlov AV, Burenin AG, Massarskaya NG, Betin AV, Nikitin MP, Nikitin PI (2017) Highly reproducible and sensitive detection of mycotoxins by label-free biosensors. Sensors Actuators B Chem 246:1080–1084. https://doi.org/10.1016/j.snb.2016.12.071
Orlov AV, Nikitin MP, Bragina VA, Znoyko SL, Zaikina MN, Ksenevich TI, Gorshkov BG, Nikitin PI (2015) A new real-time method for investigation of affinity properties and binding kinetics of magnetic nanoparticles. J Magn Magn Mater 380:231–235. https://doi.org/10.1016/j.jmmm.2014.10.019
Smith JP, Martin A, Sammons DL, Striley C, Biagini R, Quinn J, Cope R, Snawder JE (2009) Measurement of methamphetamine on surfaces using surface plasmon resonance. Toxicol Mech Methods 19:416–421. https://doi.org/10.1080/15376510903114959
Andreou C, Hoonejani MR, Barmi MR, Moskovits M, Meinhart CD (2013) Rapid detection of drugs of abuse in saliva using surface enhanced raman spectroscopy and microfluidics. ACS Nano 7:7157–7164. https://doi.org/10.1021/nn402563f
Kerrigan S, Phillips J (2001) Comparison of ELISAs for opiates, methamphetamine, cocaine metabolite, benzodiazepines, phencyclidine, and cannabinoids in whole blood and urine. Clin Chem 47:540–547 http://clinchem.aaccjnls.org/content/47/3/540/tab-article-info. Accessed 9 Apr 2019
Dams R, Benijts T, Lambert W, De Leenheer A (2002) Simultaneous determination of in total 17 opium alkaloids and opioids in blood and urine by fast liquid chromatography–diode-array detection–fluorescence detection, after solid-phase extraction. J Chromatogr B 773:53–61. https://doi.org/10.1016/S1570-0232(01)00594-3
Gandhi S, Caplash N, Sharma P, Raman Suri C (2009) Strip-based immunochromatographic assay using specific egg yolk antibodies for rapid detection of morphine in urine samples. Biosens Bioelectron 25:502–505. https://doi.org/10.1016/j.bios.2009.07.018
Acknowledgments
The authors thank Prof. N.S. Osin (State Research Institute of Biological Engineering, Moscow, Russia) and Dr. A.E. Nosyrev (Department of Analytical and Forensic Toxicology of Sechenov First Moscow State Medical University) for the help with organization of the experiments. Different aspects and parts of this multidisciplinary research were partially supported by the grants of Russian Foundation for Basic Research No. 18-33-20252 (preparation, functionalization and characterization of magnetic particles and rapid immunoassay development) and Russian Science Foundation No. 16-12-10543 (development of 3-channel MPQ-readers and multiplex assays).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Petr I. Nikitin is a named inventor on the patents on MPQ.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1.90 MB)
Rights and permissions
About this article
Cite this article
Guteneva, N.V., Znoyko, S.L., Orlov, A.V. et al. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: application to the determination of drugs of abuse. Microchim Acta 186, 621 (2019). https://doi.org/10.1007/s00604-019-3726-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3726-9