Abstract
A photoelectrochemical (PEC) method was developed for the determination of dopamine. It is making use of a composite prepared from gold nanoparticles and TiO2 (type P25) and placed on a fluorine-doped tin oxide (FTO) electrode. The composites are used for photoelectrical detection with improved electron transfer efficiency for photoproduction and with improved photoelectrical conversion efficiency. This is due to the excellent electrical conductivity and surface plasmon resonance absorption by gold nanoparticles, and also by the photocatalytic effect of TiO2. Dopamine binds easily to the surface of the composites and acts as an electron donor. This electrode gives a strongly enhanced photocurrent which increases linearly in the 0.1 to 100 μM dopamine concentration range and has a 23 nM detection limit (at S/N = 3). The electrode was operated over 15 cycles of light-on and light-off states every 20 s under visible-light illumination, and the sensor indicates good stability. In addition, it is selective over several possible interferents including uric acid, L-cysteine, glutathione, ascorbic acid and glucose.

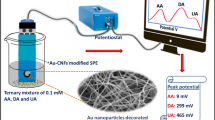
A new gold/P25 composite-based photoelectrochemical sensing scheme for dopamine is described. Under visible light irradiation, the photocurrent response is increased with the increasing concentration of dopamine (DA).







Similar content being viewed by others
Change history
01 June 2019
The published version of this article, unfortunately, contains error. The author found out that Chinese characters are shown in Scheme 1a.
References
Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM (2005) Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A 102(29):10023–10028. https://doi.org/10.1073/pnas.0504657102
Liu X, Xie L, Li H (2012) Electrochemical biosensor based on reduced graphene oxide and au nanoparticles entrapped in chitosan/silica sol–gel hybrid membranes for determination of dopamine and uric acid. J Electroanal Chem 682:158–163. https://doi.org/10.1016/j.jelechem.2012.07.031
Zhao D, Song H, Hao L, Liu X, Zhang L, Lv Y (2013) Luminescent ZnO quantum dots for sensitive and selective detection of dopamine. Talanta 107:133–139. https://doi.org/10.1016/j.talanta.2013.01.006
Qin C, Bai X, Zhang Y, Gao K (2018) Photoelectrochemical CdSe/TiO2 nanotube array microsensor for high-resolution in-situ detection of dopamine. Mikrochim Acta 185(5):278. https://doi.org/10.1007/s00604-018-2788-4
Gorwood P, Le Strat Y, Ramoz N, Dubertret C, Moalic JM, Simonneau M (2012) Genetics of dopamine receptors and drug addiction. Hum Genet 131(6):803–822. https://doi.org/10.1007/s00439-012-1145-7
Grace AA (2012) Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 62(3):1342–1348. https://doi.org/10.1016/j.neuropharm.2011.05.011
Darbin O (2012) The aging striatal dopamine function. Parkinsonism Relat D 18(5):426–432. https://doi.org/10.1016/j.parkreldis.2011.11.025
Wang HY, Feng XG, Zhang M, Zhao H (2007) Determination of dopamine in injections and urine by an enzyme-catalyzed fluorescence quenching method. Anal Sci 23(11):1297–1300
Wu B, Miao C, Yu L, Wang Z, Huang C, Jia N (2014) Sensitive electrochemiluminescence sensor based on ordered mesoporous carbon composite film for dopamine. Sensors Actuators B Chem 195:22–27. https://doi.org/10.1016/j.snb.2014.01.012
Song P, Mabrouk OS, Hershey ND, Kennedy RT (2012) In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Anal Chem 84(1):412–419. https://doi.org/10.1021/ac202794q
Habibi B, Jahanbakhshi M, Pournaghi-Azar MH (2011) Simultaneous determination of acetaminophen and dopamine using SWCNT modified carbon–ceramic electrode by differential pulse voltammetry. Electrochim Acta 56(7):2888–2894. https://doi.org/10.1016/j.electacta.2010.12.079
Liu YM, Wang CQ, Mu HB, Cao JT, Zheng YL (2007) Determination of catecholamines by CE with direct chemiluminescence detection. Electrophoresis 28(12):1937–1941. https://doi.org/10.1002/elps.200600741
Huang Y, Miao YE, Ji S, Tjiu WW, Liu T (2014) Electrospun carbon nanofibers decorated with ag-Pt bimetallic nanoparticles for selective detection of dopamine. ACS Appl Mater Interfaces 6(15):12449–12456. https://doi.org/10.1021/am502344p
Mao Y, Bao Y, Gan S, Li F, Niu L (2011) Electrochemical sensor for dopamine based on a novel graphene-molecular imprinted polymers composite recognition element. Biosens Bioelectron 28(1):291–297. https://doi.org/10.1016/j.bios.2011.07.034
Yan Y, Liu Q, Du X, Qian J, Mao H, Wang K (2015) Visible light photoelectrochemical sensor for ultrasensitive determination of dopamine based on synergistic effect of graphene quantum dots and TiO2 nanoparticles. Anal Chim Acta 853:258–264. https://doi.org/10.1016/j.aca.2014.10.021
Chen J, Gao P, Wang H, Han L, Zhang Y, Wang P, Jia N (2018) A PPy/Cu2O molecularly imprinted composite film-based visible light-responsive photoelectrochemical sensor for microcystin-LR. J Mater Chem C 6(15):3937–3944. https://doi.org/10.1039/c7tc05743a
Gao P, Wang H, Li P, Gao W, Zhang Y, Chen J, Jia N (2018) In-site synthesis molecular imprinting Nb2O5 -based photoelectrochemical sensor for bisphenol a detection. Biosens Bioelectron 121:104–110. https://doi.org/10.1016/j.bios.2018.08.070
Hun X, Wang S, Mei S, Qin H, Zhang H, Luo X (2017) Photoelectrochemical dopamine sensor based on a gold electrode modified with SnSe nanosheets. Microchim Acta 184(9):3333–3338. https://doi.org/10.1007/s00604-017-2347-4
Li H, Zhu M, Chen W, Wang K (2017) Ternary heterojunctions composed of BiOCl, BiVO4 and nitrogen-doped carbon quantum dots for use in photoelectrochemical sensing: effective charge separation and application to ultrasensitive sensing of dopamine. Microchim Acta 184(12):4827–4833. https://doi.org/10.1007/s00604-017-2529-0
Li H, Xue Y, Wang W (2014) Femtomole level photoelectrochemical aptasensing for mercury ions using quercetin-copper(II) complex as the DNA intercalator. Biosens Bioelectron 54:317–322. https://doi.org/10.1016/j.bios.2013.11.024
Wang G, Wang H, Ling Y, Tang Y, Yang X, Fitzmorris RC, Wang C, Zhang JZ, Li Y (2011) Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett 11(7):3026–3033. https://doi.org/10.1021/nl201766h
Luo J, Karuturi SK, Liu L, Su LT, Tok AI, Fan HJ (2012) Homogeneous photosensitization of complex TiO(2) nanostructures for efficient solar energy conversion. Sci Rep 2:451. https://doi.org/10.1038/srep00451
Da P, Li W, Lin X, Wang Y, Tang J, Zheng G (2014) Surface plasmon resonance enhanced real-time photoelectrochemical protein sensing by gold nanoparticle-decorated TiO(2) nanowires. Anal Chem 86(13):6633–6639. https://doi.org/10.1021/ac501406x
Hu L, Fong CC, Zhang X, Chan LL, Lam PK, Chu PK, Wong KY, Yang M (2016) Au nanoparticles decorated TiO2 nanotube arrays as a recyclable sensor for Photoenhanced electrochemical detection of bisphenol a. Environ Sci Technol 50(8):4430–4438. https://doi.org/10.1021/acs.est.5b05857
Bian Z, Tachikawa T, Zhang P, Fujitsuka M, Majima T (2014) Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J Am Chem Soc 136(1):458–465. https://doi.org/10.1021/ja410994f
Qian K, Sweeny BC, Johnston-Peck AC, Niu W, Graham JO, DuChene JS, Qiu J, Wang YC, Engelhard MH, Su D, Stach EA, Wei WD (2014) Surface plasmon-driven water reduction: gold nanoparticle size matters. J Am Chem Soc 136(28):9842–9845. https://doi.org/10.1021/ja504097v
Zhao WW, Tian CY, Xu JJ, Chen HY (2012) The coupling of localized surface plasmon resonance-based photoelectrochemistry and nanoparticle size effect: towards novel plasmonic photoelectrochemical biosensing. Chem Commun (Camb) 48(6):895–897. https://doi.org/10.1039/c1cc16775h
Zhu YC, Zhang N, Ruan YF, Zhao WW, Xu JJ, Chen HY (2016) Alkaline phosphatase tagged antibodies on gold nanoparticles/TiO2 nanotubes electrode: a Plasmonic strategy for label-free and amplified Photoelectrochemical immunoassay. Anal Chem 88(11):5626–5630. https://doi.org/10.1021/acs.analchem.6b01261
Xiao F (2012) Layer-by-layer self-assembly construction of highly ordered metal-TiO2 nanotube arrays Heterostructures (M/TNTs, M = au, ag, Pt) with tunable catalytic activities. J Phys Chem C 116(31):16487–16498. https://doi.org/10.1021/jp3034984
Gao Z-D, Han Y-Y, Li Y-C, Yang M, Song Y-Y (2014) Photocatalytic synthesis and synergistic effect of Prussian blue-decorated au nanoparticles/TiO2 nanotube arrays for H2O2 amperometric sensing. Electrochim Acta 125:530–535. https://doi.org/10.1016/j.electacta.2014.01.149
Ding IK, Zhu J, Cai W, Moon S-J, Cai N, Wang P, Zakeeruddin SM, Grätzel M, Brongersma ML, Cui Y, McGehee MD (2011) Plasmonic dye-sensitized solar cells. Adv Energy Mater 1(1):52–57. https://doi.org/10.1002/aenm.201000041
Warren SC, Thimsen E (2012) Plasmonic solar water splitting. Energy Environ Sci 5(1):5133–5146. https://doi.org/10.1039/c1ee02875h
Du J, Zhu B, Chen X (2013) Urine for plasmonic nanoparticle-based colorimetric detection of mercury ion. Small 9(24):4104–4111. https://doi.org/10.1002/smll.201300593
Thimsen E, Le Formal F, Gratzel M, Warren SC (2011) Influence of plasmonic au nanoparticles on the photoactivity of Fe(2)O(3) electrodes for water splitting. Nano Lett 11(1):35–43. https://doi.org/10.1021/nl1022354
Zhao WW, Yu XD, Xu JJ, Chen HY (2016) Recent advances in the use of quantum dots for photoelectrochemical bioanalysis. Nanoscale 8(40):17407–17414. https://doi.org/10.1039/c6nr05011e
Zhang Z, Zhang L, Hedhili MN, Zhang H, Wang P (2013) Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett 13(1):14–20. https://doi.org/10.1021/nl3029202
Archana PS, Pachauri N, Shan Z, Pan S, Gupta A (2015) Plasmonic enhancement of Photoactivity by gold nanoparticles embedded in hematite films. J Phys Chem C 119(27):15506–15516. https://doi.org/10.1021/acs.jpcc.5b02357
Wang GL, Xu JJ, Chen HY (2009) Dopamine sensitized nanoporous TiO2 film on electrodes: photoelectrochemical sensing of NADH under visible irradiation. Biosens Bioelectron 24(8):2494–2498. https://doi.org/10.1016/j.bios.2008.12.031
Cai Z, Rong M, Zhao T, Zhao L, Wang Y, Chen X (2015) Solar-induced photoelectrochemical sensing for dopamine based on TiO2 nanoparticles on g-C3N4 decorated graphene nanosheets. J Electroanal Chem 759:32–37. https://doi.org/10.1016/j.jelechem.2015.08.037
Qiao B, Liang J-X, Wang A, Xu C-Q, Li J, Zhang T, Liu JJ (2015) Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res 8(9):2913–2924. https://doi.org/10.1007/s12274-015-0796-9
Mezni A, Ibrahim MM, El-Kemary M, Shaltout AA, Mostafa NY, Ryl J, Kumeria T, Altalhi T, Amin MA (2018) Cathodically activated au/TiO2 nanocomposite synthesized by a new facile solvothermal method: an efficient electrocatalyst with Pt-like activity for hydrogen generation. Electrochim Acta 290:404–418. https://doi.org/10.1016/j.electacta.2018.08.083
Xiao FX, Zeng Z, Liu B (2015) Bridging the gap: Electron relay and Plasmonic sensitization of metal nanocrystals for metal clusters. J Am Chem Soc 137(33):10735–10744. https://doi.org/10.1021/jacs.5b06323
Baig N, Kawde A-N (2016) A cost-effective disposable graphene-modified electrode decorated with alternating layers of au NPs for the simultaneous detection of dopamine and uric acid in human urine. RSC Adv 6(84):80756–80765. https://doi.org/10.1039/c6ra10055d
Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, Kim JS, Kim H (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron 25(10):2366–2369. https://doi.org/10.1016/j.bios.2010.02.031
Yuan D, Chen S, Yuan R, Zhang J, Liu X (2014) An ECL sensor for dopamine using reduced graphene oxide/multiwall carbon nanotubes/gold nanoparticles. Sensors Actuators B Chem 191:415–420. https://doi.org/10.1016/j.snb.2013.10.013
Acknowledgements
We are grateful for financial support from the Shanghai Science and Technology Committee (17070503000), International Joint Laboratory on Resource Chemistry (IJLRC), Shanghai Engineering Research Center of Green Energy Chemical Engineering, and Program for Changjiang Scholars and Innovative Research Team in University (IRT_16R49).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Xu, M., Gao, P. et al. Photoelectrochemical sensing of dopamine using gold-TiO2 nanocomposites and visible-light illumination. Microchim Acta 186, 326 (2019). https://doi.org/10.1007/s00604-019-3401-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3401-1