Abstract
The authors describe cobalt phosphide (CoP) nanowires for use in sensitive fluorometric determination of the activity of the enzyme telomerase. A hybridization chain reaction (HCR) is applied to amplify the signal and carboxyfluorescein (FAM)-labelled hairpin probes (H1 and H2) are applied to match the telomeric DNA sequence. The CoP nanowires act as both the photoinduced electron transfer (PET) acceptor to induce fluorescence quenching, and also as an efficient probe carrier to facilitate telomerase imaging in living cells. The telomerase-triggered primer extension initiates an alternating hybridization reaction between H1 and H2. These result in the dissociation of FAM-labelled probes from CoP nanowires and thus an enhancement of the green fluorescence. The method is fairly simple and was applied to the detection of three types of cancer cells. The detection limit is as low as 7 cells (in case of HeLa cells). Conceivably, the method has a large potential in terms of inhibitor drug screening.

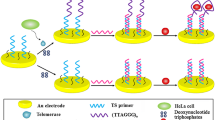
Schematic presentation of telomerase detection based on cobalt phosphide (CoP) nanowires and hybridization chain reaction (HCR). The telomerase-triggered primer extension can initiate the alternating hybridization reaction between carboxyfluorescein (FAM)-labelled hairpin probes (H1 and H2), and the generated long DNA duplex cannot be adsorbed on the CoP nanowires. This prevents the photoinduced electron transfer (PET) from FAM to CoP nanowires.









Similar content being viewed by others
References
Bryan TM, Cech TR (1999) Telomerase and the maintenance of chromosome ends. Curr Opin Cell Biol 11:318–324
Cech TR (2000) Life at the end of the chromosome: telomeres and telomerase. Angew Chem Int Ed 39:34–43
Avilion AA, Piatyszek MA, Gupta J, Shay JW, Bacchetti S, Greider CW (1996) Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res 56:645–650
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015
Shay JW, Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33:787–791
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622
Cong Y, Shay JW (2008) Actions of human telomerase beyond telomeres. Cell Res 18:725–732
Wang D, Guo R, Wei Y, Zhang Y, Zhao X, Xu Z (2018) Sensitive multicolor visual detection of telomerase activity based on catalytic hairpin assembly and etching of au nanorods. Biosens Bioelectron 122:247–253
Yu T, Zhao W, Xu J-J, Chen H-Y (2018) A PCR-free colorimetric strategy for visualized assay of telomerase activity. Talanta 178:594–599
Wang G, Wang H, Cao S, Xiang W, Li T, Yang M (2019) Electrochemical determination of the activity and inhibition of telomerase based on the interaction of DNA with molybdate. Microchim Acta 186:96–101
Wang L, Meng T, Yu G, Wu S, Sun J, Jia H, Wang H, Yang X, Zhang Y (2019) A label-free electrochemical biosensor for ultra-sensitively detecting telomerase activity based on the enhanced catalytic currents of acetaminophen catalyzed by au nanorods. Biosens Bioelectron 124:53–58
Qian R, Ding L, Yan L, Lin M, Ju H (2014) A robust probe for lighting up intracellular telomerase via primer extension to open a nicked molecular beacon. J Am Chem Soc 136:8205–8208
Xu Y, Zhang P, Wang Z, Lv S, Ding C (2018) Determination of the activity of telomerase in cancer cells by using BSA-protected gold nanoclusters as a fluorescent probe. Microchim Acta 185:198–204
Zhuang Y, Huang F, Xu Q, Zhang M, Lou X, Xia F (2016) Facile, fast-responsive, and photostable imaging of telomerase activity in living cells with a fluorescence turn-on manner. Anal Chem 88:3289–3294
Wang K, Li S, Liu Y, Jiang L, Zhang F, Wei Y, Zhang Y, Qi Z, Wang K, Liu S (2017) In situ detection and imaging of telomerase activity in cancer cell lines via disassembly of plasmonic core–satellites nanostructured probe. Anal Chem 89:7262–7268
Qian G-S, Zhang T-T, Zhao W, Xu J-J, Chen H-Y (2017) Single-molecule imaging of telomerase activity via linear plasmon rulers. Chem Commun 53:4710–4713
Kim NW, Wu F (1997) Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res 25:2595–2597
Lee NY (2018) A review on microscale polymerase chain reaction based methods in molecular diagnosis, and future prospects for the fabrication of fully integrated portable biomedical devices. Microchim Acta 185:285–291
Bi S, Ye J, Dong Y, Li H, Cao W (2016) Target-triggered cascade recycling amplification for label-free detection of microRNA and molecular logic operations. Chem Commun 52:402–405
Bi S, Cui Y, Li L (2013) Dumbbell probe-mediated cascade isothermal amplification: a novel strategy for label-free detection of microRNAs and its application to real sample assay. Anal Chim Acta 760:69–74
Ma C, Liu H, Tian T, Song X, Yu J, Yan M (2016) A simple and rapid detection assay for peptides based on the specific recognition of aptamer and signal amplification of hybridization chain reaction. Biosens Bioelectron 83:15–18
Wang W-J, Li J-J, Rui K, Gai P-P, Zhang J-R, Zhu J-J (2015) Sensitive electrochemical detection of telomerase activity using spherical nucleic acids gold nanoparticles triggered mimic-hybridization chain reaction enzyme-free dual signal amplification. Anal Chem 87:3019–3026
Hong M, Xu L, Xue Q, Li L, Tang B (2016) Fluorescence imaging of intracellular telomerase activity using enzyme-free signal amplification. Anal Chem 88:12177–12182
Yang L, Liu D, Hao S, Qu F, Ge R, Ma Y, Du G, Asiri AM, Chen L, Sun X (2017) Topotactic conversion of α-Fe2O3 nanowires into FeP as a superior fluorosensor for nucleic acid detection: insights from experiment and theory. Anal Chem 89:2191–2195
Tian J, Cheng N, Liu Q, Xing W, Sun X (2015) Cobalt phosphide nanowires: efficient nanostructures for fluorescence sensing of biomolecules and photocatalytic evolution of dihydrogen from water under visible light. Angew Chem Int Ed 54:5493–5497
Jiang P, Liu Q, Liang Y, Tian J, Asiri AM, Sun X (2014) A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew Chem Int Ed 53:12855–12859
Tang C, Gan L, Zhang R, Lu W, Jiang X, Asir AM, Sun X, Wang J, Chen L (2016) Ternary FexCo1–xP nanowire array as a robust hydrogen evolution reaction electrocatalyst with Pt-like activity: experimental and theoretical insight. Nano Lett 16:6617–6621
Jiang P, Liu Q, Sun X (2014) NiP2 Nanosheet arrays supported on carbon cloth: an efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions. Nanoscale 6:13440–13445
Tang C, Zhang R, Lu W, He L, Jiang X, Asiri AM, Sun X (2017) Fe-doped CoP nanoarray: a monolithic multifunctional catalyst for highly efficient hydrogen generation. Adv Mater 29:1602441–1602446
Li Y, Malik MA, O'Brien P (2005) Synthesis of single-crystalline CoP nanowires by a one-pot metal-organic route. J Am Chem Soc 127:16020–16021
Tian J, Liu Q, Asiri AM, Sun X (2014) Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J Am Chem Soc 136:7587–7590
Grosvenor AP, Wik SD, Cavell RG, Mar A (2005) Examination of the bonding in binary transition-metal monophosphides MP (M= Cr, Mn, Fe, co) by X-ray photoelectron spectroscopy. Inorg Chem 44:8988–8998
Li H, Yang P, Chu D, Li H (2007) Selective maltose hydrogenation to maltitol on a ternary co–P–B amorphous catalyst and the synergistic effects of alloying B and P. Appl Catal a-Gen 325:34–40
Sadava D, Whitlock E, Kane SE (2007) The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Bio Res Commun 360:233–237
Su D, Huang X, Dong C, Ren J (2018) Quantitative determination of telomerase activity by combining fluorescence correlation spectroscopy with telomerase repeat amplification protocol. Anal Chem 90:1006–1013
Acknowledgments
We gratefully acknowledge the supports from the National Natural Science Foundation of China (21775065, 21675078 and 21105044), Jiangxi Province Natural Science Foundation (20165BCB18022, 20171BAB203016 and 2018ACB21008) and National Students' innovation and entrepreneurship training program (201701075).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.75 mb)
Rights and permissions
About this article
Cite this article
Zhang, L., Peng, J., Hong, MF. et al. Cobalt phosphide nanowires for fluorometric detection and in-situ imaging of telomerase activity via hybridization chain reactions. Microchim Acta 186, 309 (2019). https://doi.org/10.1007/s00604-019-3391-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3391-z