Abstract
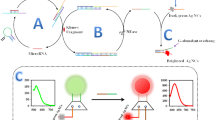
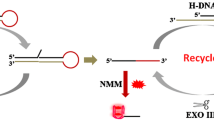
The authors describe a method for the determination of microRNA-122 by using terminal deoxynucleotidyl transferase (TdT). It is based on the use of polythymine and exonuclease III-aided cycling amplification. A 3′-phosphorylated hairpin probe 1 (H1) and a hairpin probe 2 (H2) were designed. In the presence of the microRNA, hybridization and enzymatic cleavage will occur and produce lots of 3′-hydroxylated ssDNA which can be tailed by TdT and converted into long polythymine (polyT) sequences. These can be used to synthesize copper nanoparticles (CuNPs) with fluorescence excitation/emission maxima at 350 nm/630 nm. This method shows good selectivity and high sensitivity with a linear response in the 1.00 × 102 fM and 1.00 × 106 fM microRNA concentration range and a 44 fM limit of detection. It was successfully applied to determination of microRNA in spiked serum samples.

A label-free and highly sensitive fluorometric method is described for the assay of microRNA on the basis of target-triggered two-cycle amplification and combining with terminal TdT. It produces a series superlong polyT that can be used for synthesis of copper nanoclusters (CuNCs) displaying red fluorecence.




Similar content being viewed by others
References
Fededa JP, Esk C, Mierzwa B, Stanyte R, Yuan S, Zheng H, Ebnet K, Yan W, Knoblich JA, Gerlich DW (2016) MicroRNA-34/449 controls mitotic spindle orientation during mammalian cortex development. EMBO J 35(22):2386–2398
Chang J, Davis-Dusenbery BN, Kashima R, Jiang X, Marathe N, Sessa R, Louie J, Gu W, Lagna G, Hata A (2013) Acetylation of p53 stimulates miRNA processing and determines cell survival following genotoxic stress. EMBO J 32(24):3192–3205
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866
Wang Y, Zheng D, Tan Q, Wang MX, Gu L-Q (2011) Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat Nanotechnol 6:668–674
Castoldi M, Schmidt S, Benes V, Noerholm M, Kulozik AE, Hentze MW, Muckenthaler MU (2006) A sensitive array for microRNA expression profiling (miChip) based on locked nucleic acids (LNA). RNA 12(5):913–920
Weiss FU, Marques IJ, Woltering JM, Vlecken DH, Aghdassi A, Partecke LI, Heidecke C-D, Lerch MM, Bagowski CP (2009) Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 137(6):2136–2145 e2131–2137
Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DSB (2011) Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem 57(1):84–91
Kaplan M, Kilic T, Guler G, Mandli J, Amine A, Ozsoz M (2017) A novel method for sensitive microRNA detection: Electropolymerization based doping. Biosens Bioelectron 92:770–778
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3:12
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294(5543):853–858
Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X (2013) MicroRNA: function, detection, and bioanalysis. Chem Rev 113(8):6207–6233
Wang W, Kong T, Zhang D, Zhang J, Cheng G (2015) Label-free MicroRNA detection based on fluorescence quenching of gold nanoparticles with a competitive hybridization. Anal Chem 87(21):10822–10829
Xu S, Nie Y, Jiang L, Wang J, Xu G, Wang W, Luo X (2018) Polydopamine Nanosphere/gold nanocluster (au NC)-based Nanoplatform for dual color simultaneous detection of multiple tumor-related MicroRNAs with DNase-I-assisted target recycling amplification. Anal Chem 90(6):4039–4045
Li R-D, Yin B-C, Ye B-C (2016) Ultrasensitive, colorimetric detection of microRNAs based on isothermal exponential amplification reaction-assisted gold nanoparticle amplification. Biosens Bioelectron 86:1011–1016
Miao X, Cheng Z, Ma H, Li Z, Xue N, Wang P (2018) Label-free platform for MicroRNA detection based on the fluorescence quenching of positively charged gold nanoparticles to silver nanoclusters. Anal Chem 90(2):1098–1103
Su J, Wang D, Nörbel L, Shen J, Zhao Z, Dou Y, Peng T, Shi J, Mathur S, Fan C, Song S (2017) Multicolor gold-silver Nano-mushrooms as ready-to-use SERS probes for ultrasensitive and multiplex DNA/miRNA detection. Anal Chem 89(4):2531–2538
Ding L, Liu H, Zhang L, Li L, Yu J (2018) Label-free detection of microRNA based on the fluorescence quenching of silicon nanoparticles induced by catalyzed hairpin assembly coupled with hybridization chain reaction. Sensors Actuators B Chem 254:370–376
Xu F, Shi H, He X, Wang K, He D, Guo Q, Qing Z, L a Y, Ye X, Li D, Tang J (2014) Concatemeric dsDNA-templated copper nanoparticles strategy with improved sensitivity and stability based on rolling circle replication and its application in microRNA detection. Anal Chem 86(14):6976–6982
Chi B-Z, Liang R-P, Qiu W-B, Yuan Y-H, Qiu J-D (2017) Direct fluorescence detection of microRNA based on enzymatically engineered primer extension poly-thymine (EPEPT) reaction using copper nanoparticles as nano-dye. Biosens Bioelectron 87:216–221
Xu F, Luo L, Shi H, He X, Lei Y, Tang J, He D, Qiao Z, Wang K (2018) Label-free and sensitive microRNA detection based on a target recycling amplification-integrated superlong poly(thymine)-hosted copper nanoparticle strategy. Anal Chim Acta 1010:54–61
Rotaru A, Dutta S, Jentzsch E, Gothelf K, Mokhir A (2010) Selective dsDNA-templated formation of copper nanoparticles in solution. Angew Chem Int Ed Engl 49(33):5665–5667
Qing Z, He X, He D, Wang K, Xu F, Qing T, Yang X (2013) Poly(thymine)-templated selective formation of fluorescent copper nanoparticles. Angew Chem Int Ed Engl 52(37):9719–9722
Song C, Yang X, Wang K, Wang Q, Huang J, Liu J, Liu W, Liu P (2014) Label-free and non-enzymatic detection of DNA based on hybridization chain reaction amplification and dsDNA-templated copper nanoparticles. Anal Chim Acta 827:74–79
Qiu S, Li X, Xiong W, Xie L, Guo L, Lin Z, Qiu B, Chen G (2013) A novel fluorescent sensor for mutational p53 DNA sequence detection based on click chemistry. Biosens Bioelectron 41:403–408
Hu R, Liu Y-R, Kong R-M, Donovan MJ, Zhang X-B, Tan W, Shen G-L, Yu R-Q (2013) Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label free nuclease enzymedetection. Biosens Bioelectron 42:31–35
Zhang H, Lin Z, Su X (2015) Label-free detection of exonuclease III by using dsDNA–templated copper nanoparticles as fluorescent probe. Talanta 131:59–63
Yang X-H, Sun S, Liu P, Wang K-M, Wang Q, Liu J-B, Huang J, He L-L (2014) A novel fluorescent detection for PDGF-BB based on dsDNA-templated copper nanoparticles. Chin Chem Lett 25(1):9–14
Wang H-B, Zhang H-D, Chen Y, Huang K-J, Liu Y-M (2015) A label-free and ultrasensitive fluorescent sensor for dopamine detection based on double-stranded DNA templated copper nanoparticles. Sensors Actuators B Chem 220:146–153
Liu R, Wang C, Hu J, Su Y, Lv Y (2018) DNA-templated copper nanoparticles: versatile platform for label-free bioassays. TrAC Trends Anal Chem 105:436–452
Wang G, Wan J, Zhang X (2017) TTE DNA–cu NPs: enhanced fluorescence and application in a target DNA triggered dual-cycle amplification biosensor. Chem Commun 53(41):5629–5632
Dong Z-Z, Zhang L, Qiao M, Ge J, Liu A-L, Li Z-H (2016) A label-free assay for T4 polynucleotide kinase/phosphatase activity and its inhibitors based on poly(thymine)-templated copper nanoparticles. Talanta 146:253–258
He Y, Jiao B (2018) Detection of biotin-streptavidin interactions based on poly(thymine)-templated copper nanoparticles coupled with Exo III-aided DNA recycling amplification. Sensors Actuators B Chem 265:387–393
Song Q, Wang R, Sun F, Chen H, Wang Z, Na N, Ouyang J (2017) A nuclease-assisted label-free aptasensor for fluorescence turn-on detection of ATP based on the in situ formation of copper nanoparticles. Biosens Bioelectron 87:760–763
Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP (2006) miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 3(2):87–98
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors got the permission for using serum sample of human volunteers from Guilin’s Fifth People’s Hospital (China) according to institutional guidelines. All animal procedures and all experiments were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Guangxi Normal University (Guilin, China) and approved by the Animal Ethics Committee of China.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2.15 mb)
Rights and permissions
About this article
Cite this article
Tang, Y., Liu, M., Zhao, Z. et al. Fluorometric determination of microRNA-122 by using ExoIII-aided recycling amplification and polythymine induced formation of copper nanoparticles. Microchim Acta 186, 133 (2019). https://doi.org/10.1007/s00604-019-3237-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3237-8