Abstract
The authors describe an isothermal and ultrasensitive colorimetric DNA assay that consists of two amplification stages using enzymes and a catalytic hairpin assembly (CHA). The first step consists in the selective amplification of DNA using Klenow fragment and nicking enzyme. The second step consists in the amplification of the optical signal by using a catalytic hairpin assembly. After two amplification steps, the DNA reaction induces the aggregation of the red gold nanoparticles to give a blue color shift. The degree of aggregation can be quantified by measurement of the ratio of the UV-vis absorbances of the solutions at 620 and 524 nm which are the wavelengths of the aggregated gold nanoparticles and bare gold nanoparticles. The detection limit is as low as 3.1 fM. Due to the use of a specific enzyme, only the desired DNAs will be detected. The method can be applied to the determination of DNA of various lengths. Despite the presence of large amounts of wildtype DNA, it can readily detect a target DNA. Conceivably, the technique has a large potential because of its high sensitivity and selectivity.

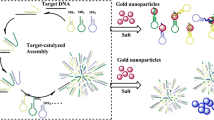
Schematic presentation of DNA detection using gold nanoparticles (AuNP), enzymes and catalytic hairpin assembly (CHA). Effective DNA detection is achieved through the aggregation of AuNPs which is caused by DNA amplification using enzymes and signal amplification using CHA.






Similar content being viewed by others
References
Jang K, Choi J, Park C, Na S (2017) Label-free and high-sensitive detection of Kirsten rat sarcoma viral oncogene homolog and epidermal growth factor receptor mutation using kelvin probe force microscopy. Biosens Bioelectron 87:222–228. https://doi.org/10.1016/j.bios.2016.08.045
Yatabe Y, Hida T, Horio Y, Kosaka T, Takahashi T, Mitsudomi T (2006) A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung Cancer. The Journal of Molecular Diagnostics 8(3):335–341. https://doi.org/10.2353/jmoldx.2006.050104
Dena M, Kelly B, Anand K, Madelyn SL, Michael M, Franziska B, Katharina V, Daniel L, Jorge N, Lyudmila B, Andrew HK, Korn WM, Ethan S, Michael C, Xing Y, Thomas M, Rachelle L, Meghana H, Craig Y, Joshua K, Yelena P, Devin S, David N, Peter K (2012) Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 9(1):016003
Marco W, Lyudmila B, Rogier B, Anand K, Meghana H, Edward HC, Dena M, Ajay S, Anthony P, Patricia T, Kelly B, Jorge N, Michel van den H, Peter K (2012) Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol 9(1):016005
Taly V, Pekin D, Benhaim L, Kotsopoulos SK, Le Corre D, Li X, Atochin I, Link DR, Griffiths AD, Pallier K, Blons H, Bouché O, Landi B, Hutchison JB, Laurent-Puig P (2013) Multiplex Picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal Cancer patients. Clin Chem 59(12):1722–1731. https://doi.org/10.1373/clinchem.2013.206359
Tuononen K, Mäki-Nevala S, Sarhadi VK, Wirtanen A, Rönty M, Salmenkivi K, Andrews JM, Telaranta-Keerie AI, Hannula S, Lagström S, Ellonen P, Knuuttila A, Knuutila S (2013) Comparison of targeted next-generation sequencing (NGS) and real-time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin-fixed, paraffin-embedded tumor material of non-small cell lung carcinoma—superiority of NGS. Genes Chromosom Cancer 52(5):503–511. https://doi.org/10.1002/gcc.22047
Richardson AL, Iglehart JD (2012) BEAMing up personalized medicine: mutation detection in blood. Clin Cancer Res 18(12):3209–3211. https://doi.org/10.1158/1078-0432.ccr-12-0871
Xu X-W, Weng X-H, Wang C-L, Lin W-W, Liu A-L, Chen W, Lin X-H (2016) Detection EGFR exon 19 status of lung cancer patients by DNA electrochemical biosensor. Biosens Bioelectron 80:411–417. https://doi.org/10.1016/j.bios.2016.02.009
Wu C-C, Ko F-H, Yang Y-S, Hsia D-L, Lee B-S, Su T-S (2009) Label-free biosensing of a gene mutation using a silicon nanowire field-effect transistor. Biosens Bioelectron 25(4):820–825. https://doi.org/10.1016/j.bios.2009.08.031
Liu Q, Yin Lim S, Soo RA, Kyoung Park M, Shin Y (2015) A rapid MZI-IDA sensor system for EGFR mutation testing in non-small cell lung cancer (NSCLC). Biosens Bioelectron 74:865–871. https://doi.org/10.1016/j.bios.2015.07.055
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277(5329):1078–1081. https://doi.org/10.1126/science.277.5329.1078
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL (1998) One-pot colorimetric differentiation of polynucleotides with Single Base imperfections using gold nanoparticle probes. J Am Chem Soc 120(9):1959–1964. https://doi.org/10.1021/ja972332i
Zhao W, Lam JCF, Chiuman W, Brook MA, Li Y (2008) Enzymatic cleavage of nucleic acids on gold nanoparticles: a generic platform for facile colorimetric biosensors. Small 4(6):810–816. https://doi.org/10.1002/smll.200700757
Ma H, Li Z, Xue N, Cheng Z, Miao X (2018) A gold nanoparticle based fluorescent probe for simultaneous recognition of single-stranded DNA and double-stranded DNA. Microchim Acta 185(2):93
Zeng Y, Zhang D, Qi P, Zheng L (2017) Colorimetric detection of DNA by using target catalyzed DNA nanostructure assembly and unmodified gold nanoparticles. Microchim Acta 184(12):4809–4815
Liang C, Chu Y, Cheng S, Wu H, Kajiyama T, Kambara H, Zhou G (2012) Multiplex loop-mediated isothermal amplification detection by sequence-based barcodes coupled with nicking endonuclease-mediated pyrosequencing. Anal Chem 84(8):3758–3763. https://doi.org/10.1021/ac3003825
Zhang Y, Hu J, C-y Z (2012) Sensitive detection of transcription factors by isothermal exponential amplification-based colorimetric assay. Anal Chem 84(21):9544–9549. https://doi.org/10.1021/ac3024087
Zhao Y, Chen F, Wu Y, Dong Y, Fan C (2013) Highly sensitive fluorescence assay of DNA methyltransferase activity via methylation-sensitive cleavage coupled with nicking enzyme-assisted signalamplification. Biosens Bioelectron 42:56–61. https://doi.org/10.1016/j.bios.2012.10.022
Cui L, Ke G, Zhang WY, Yang CJ (2011) A universal platform for sensitive and selective colorimetric DNA detection based on Exo III assisted signal amplification. Biosens Bioelectron 26(5):2796–2800. https://doi.org/10.1016/j.bios.2010.11.005
Xu W, Xue X, Li T, Zeng H, Liu X (2009) Ultrasensitive and selective colorimetric DNA detection by nicking endonuclease assisted nanoparticle amplification. Angew Chem Int Ed 48(37):6849–6852. https://doi.org/10.1002/anie.200901772
Dirks RM, Pierce NA (2004) Triggered amplification by hybridization chain reaction. Proc Natl Acad Sci U S A 101(43):15275–15278. https://doi.org/10.1073/pnas.0407024101
Park C, Song Y, Jang K, Choi C-H, Na S (2018) Target switching catalytic hairpin assembly and gold nanoparticle colorimetric for EGFR mutant detection. Sensors Actuators B Chem 261:497–504. https://doi.org/10.1016/j.snb.2018.01.183
Li B, Jiang Y, Chen X, Ellington AD (2012) Probing spatial organization of DNA strands using enzyme-free hairpin assembly circuits. J Am Chem Soc 134(34):13918–13921. https://doi.org/10.1021/ja300984b
Zheng A-X, Li J, Wang J-R, Song X-R, Chen G-N, Yang H-H (2012) Enzyme-free signal amplification in the DNAzyme sensor via target-catalyzed hairpin assembly. Chem Commun 48(25):3112–3114
Xu F, Dong H, Cao Y, Lu H, Meng X, Dai W, Zhang X, Al-Ghanim KA, Mahboob S (2016) Ultrasensitive and multiple disease-related MicroRNA detection based on tetrahedral DNA nanostructures and duplex-specific nuclease-assisted signal amplification. ACS Appl Mater Interfaces 8(49):33499–33505. https://doi.org/10.1021/acsami.6b12214
Yang W, Zhou X, Zhao J, Xu W (2018) A cascade amplification strategy of catalytic hairpin assembly and hybridization chain reaction for the sensitive fluorescent assay of the model protein carcinoembryonic antigen. Microchim Acta 185(2):100. https://doi.org/10.1007/s00604-017-2620-6
Yue S, Zhao T, Qi H, Yan Y, Bi S (2017) Cross-catalytic hairpin assembly-based exponential signal amplification for CRET assay with low background noise. Biosens Bioelectron 94:671–676. https://doi.org/10.1016/j.bios.2017.03.071
Zhuang J, Lai W, Chen G, Tang D (2014) A rolling circle amplification-based DNA machine for miRNA screening coupling catalytic hairpin assembly with DNAzyme formation. Chem Commun 50(22):2935–2938. https://doi.org/10.1039/C3CC49873E
Pantel K, Alix-Panabières C (2013) Real-time liquid biopsy in Cancer patients: fact or fiction? Cancer Res 73:6384–6388. https://doi.org/10.1158/0008-5472.can-13-2030
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) under grant numbers of NRF-2016R1A5A1010148, NRF-2015M3A9D7031026 and funded by the Ministry of Science, ICT & Future Planning. C. Park and H. Park contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 341 kb)
Rights and permissions
About this article
Cite this article
Park, C., Park, H., Lee, H.J. et al. Double amplified colorimetric detection of DNA using gold nanoparticles, enzymes and a catalytic hairpin assembly. Microchim Acta 186, 34 (2019). https://doi.org/10.1007/s00604-018-3154-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3154-2