Abstract
The authors describe a fluorometric aptamer based assay for detecting β-lactoglobulin by using carbon dots (C-dots) as a signal indicator. The aptamer was immoblized on magnetite (Fe3O4) nanoparticles (MNPs), and the C-dots served as a label for the complementary oligonucleotide (cDNA). The assay is based on the hybridization that takes place between aptamer and cDNA. In the presence of β-lactoglobulin (β-LG), the aptamer preferentially binds to β-LG, and this leads to a partial release of the C-dots-cDNA into the solution. After magnetic separation, the supernatant of the solution contains the released C-dots-cDNA which are quantified by fluorometry, best under excitation/emission wavelengths of 354/447 nm. Under the optimal conditions, the fluorescence intensity is proportional to the logarithm of the β-LG concentration in the 0.25 to 50 ng mL−1 range, with a 37 pg mL−1 detection limit. The method was successfully applied to the determination of β-LG in hypoallergenic formulations, and the results demonstrated that this assay is a promising tool in food quality control. Conceivably, it also provides the opportunity for detection of other analytes.

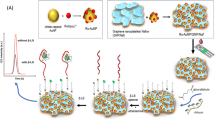
Schematic of a novel aptamer based fluorometric β-lactoglobulin assay based on the use of magnetite (Fe3O4) nanoparticles (MNPs) and carbon dots (C-dots). C-dots were used as a signal indicator and Fe3O4 MNPs acted as a magnetic separator. This assay exhibits high sensitivity and selectivity with a detection limit as low as 37 pg mL−1.






Similar content being viewed by others
References
Eissa S, L'Hocine L, Siaj M, Zourob M (2013) A graphene-based label-free voltammetric immunoassay for sensitive detection of the egg allergen ovalbumin. Analyst 138:4378–4384. https://doi.org/10.1039/c3an36883a
Lifschitz C, Szajewska H (2015) Cow's milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr 174:141–150. https://doi.org/10.1007/s00431-014-2422-3
Exl BM (2001) A review of recent developments in the use of moderately hydrolyzed whey formulae in infant nutrition. Nutr Res 21:355–379. https://doi.org/10.1016/S0271-5317(00)00259-1
Besler M, Steinhart H, Paschke A (2001) Stability of food allergens and allergenicity of processed foods. J Chromatogr B 756(2001):207–228. https://doi.org/10.1016/S0378-4347(01)00110-4
Ren Y, Han Z, Chu X, Zhang J, Cai Z, Wu Y (2010) Simultaneous determination of bovine α-lactalbumin and β-lactoglobulin in infant formulae by ultra-high-performance liquid chromatography-mass spectrometry. Anal Chim Acta 667:96–102. https://doi.org/10.1016/j.aca.2010.04.015
Billakanti JM, Fee CJ, Lane FR, Kash AS, Fredericks R (2010) Simultaneous, quantitative detection of five whey proteins in multiple samples by surface plasmon resonance. Int Dairy J 20:96–105. https://doi.org/10.1016/j.idairyj.2009.08.008
Wu X, Li Y, Liu B, Feng Y, He W, Liu Z, Liu L, Wang Z, Huang H (2015) Two-site antibody Immunoanalytical detection of food allergens by surface Plasmon resonance. Food Anal Methods 9:582–588. https://doi.org/10.1007/s12161-015-0232-5
Eissa S, Tlili C, L'Hocine L, Zourob M (2012) Electrochemical immunoassay for the milk allergen β-lactoglobulin based on electrografting of organic film on graphene modified screen-printed carbon electrodes. Biosens Bioelectron 38:308–313. https://doi.org/10.1016/j.bios.2012.06.008
Ruiz-Valdepeñas Montiel V, Campuzano S, Conzuelo F, Torrente-Rodríguez RM, Gamella M, Reviejo AJ, Pingarrón JM (2015) Electrochemical magnetoimmunosensing platform for determination of the milk allergen β-lactoglobulin. Talanta 131:156–162. https://doi.org/10.1016/j.talanta.2014.07.076
He S, Li X, Gao J, Tong P, Chen H (2017) Development of a H2O2-sensitive quantum dots-based fluorescent sandwich ELISA for sensitive detection of bovine β-lactoglobulin by monoclonal antibody. J Sci Food Agric doi. https://doi.org/10.1002/jsfa.8489
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49:6726–6744. https://doi.org/10.1002/anie.200906623
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381. https://doi.org/10.1039/c4cs00269e
Miao H, Wang L, Zhuo Y, Zhou Z, Yang X (2016) Label-free fluorimetric detection of CEA using carbon dots derived from tomato juice. Biosens Bioelectron 86:83–89. https://doi.org/10.1016/j.bios.2016.06.043
Wu Y, Wei P, Pengpumkiat S, Schumacher EA, Remcho VT (2015) Development of a carbon dot (C-dot)-linked immunosorbent assay for the detection of human α-fetoprotein. Anal Chem 87:8510–8516. https://doi.org/10.1021/acs.analchem.5b02019
Zou C, Foda MF, Tan X, Shao K, Wu L, Lu Z, Bahlol HS, Han H (2016) Carbon-dot and quantum-dot-coated dual-emission Core-satellite silica nanoparticles for Ratiometric intracellular Cu2+ imaging. Anal Chem 88:7395–7403. https://doi.org/10.1021/acs.analchem.6b01941
Zhou Y, Huang X, Liu C, Zhang R, Gu X, Guan G, Jiang C, Zhang L, Du S, Liu B, Han MY, Zhang Z (2016) Color-multiplexing-based fluorescent test paper: dosage-sensitive visualization of arsenic(III) with discernable scale as low as 5 ppb. Anal Chem 88:6105–6109. https://doi.org/10.1021/acs.analchem.6b01248
Wang H, Lu Q, Hou Y, Liu Y, Zhang Y (2016) High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 155:62–69. https://doi.org/10.1016/j.talanta.2016.04.020
Huang Y, Zhou J, Feng H, Zheng J, Ma HM, Liu W, Tang C, Ao H, Zhao M, Qian Z (2016) A dual-channel fluorescent chemoassay for discriminative detection of glutathione based on functionalized carbon quantum dots. Biosens Bioelectron 86:748–755. https://doi.org/10.1016/j.bios.2016.07.081
Osborne SE, Ellington AD (1997) Nucleic acid selection and the challenge of combinatorial chemistry. Chem Rev 97:349–370. https://doi.org/10.1021/cr960009c
Crivianu-Gaita V, Thompson M (2016) Aptamers, antibody scFv, and antibody Fab’ fragments: an overview and comparison of three of the most versatile bioassay biorecognition elements. Biosens Bioelectron 85:32–45. https://doi.org/10.1016/j.bios.2016.04.091
Famulok M, Mayer G (2011) Aptamer modules as assays and detectors. Acc Chem Res 44:1349–1358. https://doi.org/10.1021/ar2000293
Shahdost-Fard F, Roushani M (2016) Conformation switching of an aptamer based on cocaine enhancement on a surface of modified GCE. Talanta 154:7–14. https://doi.org/10.1016/j.talanta.2016.03.055
Huang H, Shi S, Gao X, Gao R, Zhu Y, Wu X, Zang R, Yao T (2016) A universal label-free fluorescent aptaassay based on Ru complex and quantum dots for adenosine, dopamine and 17β-estradiol detection. Biosens Bioelectron 79:198–204. https://doi.org/10.1016/j.bios.2015.12.024
Eissa S, Zourob M (2017) In vitro selection of DNA aptamers targeting β-lactoglobulin and their integration in graphene-based bioassay for the detection of milk allergen. Biosens Bioelectron 91:169–174. https://doi.org/10.1016/j.bios.2016.12.020
Xiao D, Lu T, Zeng R, Bi Y (2016) Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta 183:2655–2675. https://doi.org/10.1007/s00604-016-1928-y
Deng Y, Qi D, Deng C, Zhang X, Zhao D (2008) Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 Core and perpendicularly aligned Mesoporous SiO2 Shell for removal of microcystins. J Am Chem Soc 130:28–29. https://doi.org/10.1021/ja0777584
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, assays, and bioimaging. Angew Chem Int Ed 52:3953–3957. https://doi.org/10.1002/anie.201300519
Zhang H, Chen Y, Liang M, Xu L, Qi S, Chen H, Chen X (2014) Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal Chem 86:9846–9852. https://doi.org/10.1021/ac502446m
Dong Y, Wang R, Li G, Chen C, Chi Y, Chen G (2012) Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 50:2810–2815. https://doi.org/10.1021/ac3012126
Jiang Q, Zhang D, Cao Y, Gan N (2017) An antibody-free and signal-on type electrochemiluminescence assay for diethylstilbestrol detection based on magnetic molecularly imprinted polymers-quantum dots labeled aptamer conjugated probes. J Electroanal Chem 789:1–8. https://doi.org/10.1016/j.jelechem.2017.02.020
Alibolandi M, Hadizadeh F, Vajhedin F, Abnous K (2015) Ramezani M design and fabrication of an aptaassay for chloramphenicol based on energy transfer of CdTe quantum dots to graphene oxide sheet. Mater Sci Eng C 48:611–619. https://doi.org/10.1016/j.msec.2014.12.052
Dong H, Gao W, Yan F, Ji H, Ju H (2010) Fluorescence resonance energy transfer between quantum dots and Graphene oxide for sensing biomolecules. Anal Chem 82:5511–5517. https://doi.org/10.1021/ac100852z
Liu H, Xu S, He Z, Deng A, Zhu JJ (2013) Supersandwich Cytoassay for selective and ultrasensitive detection of cancer cells using Aptamer-DNA Concatamer-quantum dots probes. Anal Chem 85:3385–3392. https://doi.org/10.1021/ac303789x
He S, Huang BH, Tan J, Luo QY, Lin Y, Li J, Hu Y, Zhang L, Yan S, Zhang Q, Pang DW, Li L (2011) One-to-one quantum dot-labeled single long DNA probes. Biomaterials 32:5471–5477. https://doi.org/10.1016/j.biomaterials.2011.04.013
Knipping K, van Roest M, Kruijssen L, Smits M, Teunis M, Cox L, de Jong N, Simons PJ, Boon L, Teshima R, Gros M, Kegler D, Garssen J, Knippels LM, Pieters R (2016) Intra- and inter-laboratory validation of an innovative huFcεRIα-RBL-2H3 degranulation assay for in vitro allergenicity assessment of whey hydrolysates. Toxicol in Vitro 33:29–34. https://doi.org/10.1016/j.tiv.2016.02.018
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 61775099) and Natural Science Foundation of Jiangsu Province (No. BK20171487).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 261 kb)
Rights and permissions
About this article
Cite this article
Shi, M., Cen, Y., Sohail, M. et al. Aptamer based fluorometric β-lactoglobulin assay based on the use of magnetic nanoparticles and carbon dots. Microchim Acta 185, 40 (2018). https://doi.org/10.1007/s00604-017-2569-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2569-5