Abstract
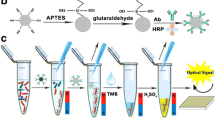
Vibrio parahaemolyticus (V. parahaemolyticus) is one of the most common food-borne pathogens. The authors describe a rapid colorimetric assay for V. parahaemolyticus that is based on a combination of a magnetic bead-based sandwich immunoassay and signal amplification via an enzmye mimic. MnO2 nanoparticles are used as an artificial oxidase that oxidizes 3,3′,5,5′-tetramethylbenzidine in the presence of oxygen to form a blue (and readily visible) product with an absorption maximum at 652 nm. By combining the superior capture efficiency of magnetic beads with the high catalytic activity of the enzmye mimic, this method can detect V. parahaemolyticus concentration in the range between 10 to 105 cfu·mL−1 without pre-enrichment, and the limit of detection is as low as 10 cfu·mL−1. Recoveries ranging from 87.5% to 106.0% are found when analyzing spiked oyster samples. The assay is rapid, sensitive, and specific and specific. In our perception, it shows promise in rapid instrumental and on-site visual detection of V. parahaemolyticus.

Schematic of colorimetric immunoassay for Vibrio parahaemolyticus detection that combines the superior capture efficiency of magnetic beads with the high catalytic activity of an enzyme mimic. The visual detection method shows high sensitivity and specificity.




Similar content being viewed by others
References
Huehn S, Eichhorn C, Urmersbach S, Breidenbach J, Bechlars S, Bier N, Alter T, Bartelt E, Frank C, Oberheitmann B, Gunzer F, Brennholt N, Böer S, Appel B, Dieckmann R, Strauch E (2014) Pathogenic vibrios in environmental, seafood and clinical sources in Germany. Int J Med Microbiol 304(7):843–850
Su YC, Liu C (2007) Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 24(6):549–558
Broberg CA, Calder TJ, Orth K (2011) Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect 13(12-13):992–1001
DePaola A, Kaysner CA, Bowers J, Cook DW (2000) Environmental Investigations of Vibrio parahaemolyticus in Oysters after Outbreaks in Washington, Texas, and New York (1997 and 1998). Appl Environ Microbiol 66(11):4649–4654
Drake SL, DePaola A, Jaykus LA (2007) An Overview of Vibrio vulnificus and Vibrio parahaemolyticus. Compr Rev Food Sci F 6(4):120–144
Di H, Ye L, Neogi SB, Meng H, Yan H, Yamasaki S, Shi L (2015) Development and evaluation of a loop-mediated isothermal amplification assay combined with enrichment culture for rapid detection of very low numbers of Vibrio parahaemolyticus in seafood samples. Biol Pharm Bull 38(1):82–87
Park B, Choi SJ (2017) Sensitive immunoassay-based detection of Vibrio parahaemolyticus using capture and labeling particles in a stationary liquid phase lab-on-a-chip. Biosens Bioelectron 90:269–275
Zhang Z, Xiao L, Lou Y, Jin M, Liao C, Malakar PK, Pan Y, Zhao Y (2015) Development of a multiplex real-time PCR method for simultaneous detection of Vibrio parahaemolyticus, Listeria monocytogenes and Salmonella spp. in raw shrimp. Food Control 51:31–36
Niemz A, Ferguson TM, Boyle DS (2011) Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol 29(5):240–250
Cho IH, Mauer L, Irudayaraj J (2014) In-situ fluorescent immunomagnetic multiplex detection of foodborne pathogens in very low numbers. Biosens Bioelectron 57:143–148
Shukla S, Lee G, Song X, Park S, Kim M (2016) Immunoliposome-based immunomagnetic concentration and separation assay for rapid detection of Cronobacter sakazakii. Biosens Bioelectron 77:986–994
Chattopadhyay S, Kaur A, Jain S, Sabharwal PK, Singh H (2016) Functionalized polymeric magnetic nanoconstructs for selective capturing and sensitive detection of Salmonella typhimurium. Anal Chim Acta 937:127–135
Wu S, Wang Y, Duan N, Ma H, Wang Z (2015) Colorimetric aptasensor based on enzyme for the detection of Vibrio parahemolyticus. J Agric Food Chem 63(35):7849–7854
Liu J, Meng L, Fei Z, Dyson PJ, Jing X, Liu X (2017) MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens Bioelectron 57:69–74
Liu X, Wang Q, Zhao H, Zhang L, Su Y, Lv Y (2012) BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst 137(19):4552–4558
Yuan J, Cen Y, Kong XJ, Wu S, Liu CL, Yu RQ, Chu X (2015) MnO2-Nanosheet-Modified Upconversion Nanosystem for Sensitive Turn-On Fluorescence Detection of H2O2 and Glucose in Blood. ACS Appl Mater Interfaces 7(19):10548–10555
Yu H, Zheng L (2016) Manganese dioxide nanosheets as an optical probe for photometric determination of free chlorine. Microchim Acta 183(7):2229–2234
Liu Y, Zhao C, Fu K, Song X, Xu K, Wang J, Li J (2017) Selective turn-on fluorescence detection of Vibrio parahaemolyticus in food based on charge-transfer between CdSe/ZnS quantum dots and gold nanoparticles. Food Control 80:380–387
Song D, Qu X, Liu Y, Li L, Yin D, Li J, Xu K, Xie R, Zhai Y, Zhang H, Bao H, Zhao C, Wang J, Song X, Song W (2017) A Rapid Detection Method of Brucella with Quantum Dots and Magnetic Beads Conjugated with Different Polyclonal Antibodies. Nanoscale Res Lett 12(1):179
Fan K, Wang H, Xi J, Liu Q, Meng X, Duan D, Gao L, Yan X (2016) Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun 53(2):424–427
Li F, Zhao Q, Wang C, Lu X, Li XF, Le XC (2010) Detection of Escherichia coli O157:H7 using gold nanoparticle labeling and inductively coupled plasma mass spectrometry. Anal Chem 82(8):3399–3403
Wen CY, Jiang YZ, Li XY, Tang M, Wu LL, Hu J, Pang DW, Zeng JB (2017) Efficient Enrichment and Analyses of Bacteria at Ultralow Concentration with Quick-Response Magnetic Nanospheres. ACS Appl Mater Interfaces 9(11):9416–9425
Retnakumari A, Setua S, Menon D, Ravindran P, Muhammed H, Pradeep T, Nair S, Koyakutty M (2010) Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 21(5):055103
Wang W, Wang Z, Liu J, Luo Z, Suib SL, He P, Ding G, Zhang Z, Sun L (2017) Single-step One-pot Synthesis of TiO2 Nanosheets Doped with Sulfur on Reduced Graphene Oxide with Enhanced Photocatalytic Activity. Sci Rep 7:46610
Gao Z, Xu M, Hou L, Chen G, Tang D (2013) Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal Chim Acta 776:79–86
Dávalos-Pantoja L, Ortega-Vinuesa JL, Bastos-González D, Hidalgo-Alvarez R (2000) A comparative study between the adsorption of IgY and IgG on latex particles. J Biomater Sci Polym Ed 11(6):657–673
Duan N, Yan Y, Wu S, Wang Z (2016) Vibrio parahaemolyticus detection aptasensor using surface-enhanced Raman scattering. Food Control 63:122–127
Teng J, Ye Y, Yao L, Yan C, Cheng K, Xue F, Pan D, Li B, Chen W (2017) Rolling circle amplification based amperometric aptamer/immuno hybrid biosensor for ultrasensitive detection of Vibrio parahaemolyticus. Microchim Acta 184(9):3477–3485
Duan N, Shen M, Wu S, Zhao C, Ma X, Wang Z (2017) Graphene oxide wrapped Fe3O4@Au nanostructures as substrates for aptamer-based detection of Vibrio parahaemolyticus by surface-enhanced Raman spectroscopy. Microchim Acta 184(8):2653–2660
Acknowledgements
All the authors acknowledge the Jilin University shared instrumentation facility, and School of Public Health, Jilin University for providing bacteria. This work was supported by the Chinese National Natural Science Foundation (Grant No. 81473018, 81602894, and 81602895) and China Postdoctoral Science Foundation (2017 T100214, 2016 M591492).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1750 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Zhao, C., Song, X. et al. Colorimetric immunoassay for rapid detection of Vibrio parahaemolyticus . Microchim Acta 184, 4785–4792 (2017). https://doi.org/10.1007/s00604-017-2523-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2523-6