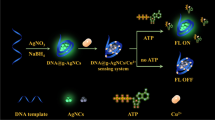
Abstract
The authors describe a biosensor for histidine that is based on the use of a DNAzyme catalytic beacon. The Cu(II)-dependent DNA-cleaving DNAzyme (Cu-Enzyme) was modified with a quencher (BHQ1) at its 5′ end, and the corresponding substrate strand (Cu-Sub) was modified with a quencher and the FAM fluorophore at its 5′ and 3′ ends, respectively. The green FAM emission of the system is completely quenched after the Cu-Enzyme is hybridized with Cu-Sub. The presence of Cu(II) triggers the cleavage of the Cu-Sub so that fluorescence recovers. Histidine forms a complex with Cu(II) ion. The complex is not capable of cleaving Cu-Sub effectively so that the fluorescence of the system is not restored. These findings were exploited to design a robust and sensitive assay for the determination of histidine. Fluorescence intensity is linearly related to the concentration of histidine in the range between 0.05 and 40 μM, and the detection limit is 20 nM. The method has been successfully applied to the determination of histidine in (spiked) human urine and gave satisfying results.

Schematic of a fluorescence biosensor for histidine based on a quencher-labeled Cu-Enzyme (Cu-Enzyme refers to Cu(II)-dependent DNA-cleaving DNAzyme; Cu-Sub refers to the corresponding substrate strand of Cu-Enzyme: Cu-Substrate; SA refers to sodium ascorbate).




Similar content being viewed by others
References
Bessho Y, Iwakoshi-Ukena E, Tachibana T, Maejima S, Taniuchi S, Masuda K, Shikano K, Kondo K, Furumitsu M, Ukena K (2014) Characterization of an avian histidine decarboxylase and localization of histaminergic neurons in the chicken brain. Neurosci Lett 578:106–110. doi:10.1016/j.neulet.2014.06.048
Zhao F, Ghezzo-Schöneich E, Aced GI, Hong J, Milby T, Schöneich C (1997) Metal-catalyzed oxidation of histidine in human growth hormone mechanism, isotope effects, and inhibition by a mild denaturing alcohol. J Biol Chem 272(14):9019–9029. doi:10.1074/jbc.272.14.9019
Jones AL, Hulett MD, Parish CR (2005) Histidine-rich glycoprotein: a novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol Cell Biol 83(2):106–118. doi:10.1111/j.1440-1711.2005.01320.x
Ma DL, Wong WL, Chung WH, Chan FY, So PK, Lai TS, Zhou ZY, Leung YC, Wong KY (2008) A highly selective luminescent switch-on probe for histidine/histidine-rich proteins and its application in protein staining. Angew Chem 120(20):3795–3799. doi:10.1002/ange.200705319
Watanabe M, Suliman ME, Qureshi AR, Garcia-Lopez E, Bárány P, Heimbürger O, Stenvinkel P, Lindholm B (2008) Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Am J Clin Nutr 87(6):1860–1866
Gerber DA (1975) Low free serum histidine concentration in rheumatoid arthritis. A measure of disease activity. J Clin Invest 55(6):1164. doi:10.1172/JCI108033
Rao ML, Stefan H, Scheid C, Kuttler AD, Froscher W (1993) Serum amino acids, liver status, and antiepileptic drug therapy in epilepsy. Epilepsia 34(2):347–354. doi:10.1111/j.1528-1157.1993.tb02420.x
Ensafi AA, Hajian R (2006) Determination of tryptophan and histidine by adsorptive cathodic stripping voltammetry using H-point standard addition method. Anal Chim Acta 580(2):236–243. doi:10.1016/j.aca.2006.07.076
Kurzątkowska K, Shpakovsky D, Radecki J, Radecka H, Jingwei Z, Milaeva E (2009) Iron (III) porphyrin bearing 2, 6-di-tert-butylphenol pendants deposited onto gold electrodes for amperometric determination of l-histidine. Talanta 78(1):126–131. doi:10.1016/j.talanta.2008.10.051
Deo RP, Lawrence NS, Wang J (2004) Electrochemical detection of amino acids at carbon nanotube and nickel–carbon nanotube modified electrodes. Analyst 129(11):1076–1081. doi:10.1039/B407418A
Hortalá MA, Fabbrizzi L, Marcotte N, Stomeo F, Taglietti A (2003) Designing the selectivity of the fluorescent detection of amino acids: a chemosensing ensemble for histidine. J Am Chem Soc 125(1):20–21. doi:10.1021/ja027110l
Liu Y-R, Hu R, Liu T, Zhang X-B, Tan W, Shen G-L, Yu R-Q (2013) Label-free dsDNA-Cu NPs-based fluorescent probe for highly sensitive detection of l-histidine. Talanta 107:402–407. doi:10.1016/j.talanta.2013.01.038
Li X, Ma H, Dong S, Duan X, Liang S (2004) Selective labeling of histidine by a designed fluorescein-based probe. Talanta 62(2):367–371. doi:10.1016/j.talanta. 2003.08.004
Kong R-M, Zhang X-B, Chen Z, Meng H-M, Song Z-L, Tan W, Shen G-L, Yu R-Q (2011) Unimolecular catalytic DNA biosensor for amplified detection of L-histidine via an enzymatic recycling cleavage strategy. Anal Chem 83(20):7603–7607. doi:10.1021/ac2018926
Zhang L-Y, Sun M-X (2004) Determination of histamine and histidine by capillary zone electrophoresis with pre-column naphthalene −2, 3-dicarboxaldeh- yde derivatization and fluorescence detection. J Chromatogr A 1040(1):133–140. doi:10.1016/j.chroma.2004.03.051
Meng J, Zhang W, Cao C-X, Fan L-Y, Wu J, Wang Q-L (2010) Moving affinity boundary electrophoresis and its selective isolation of histidine in urine. Analyst 135(7):1592–1599. doi:10.1039/C000472C
Tateda N, Matsuhisa K, Hasebe K, Kitajima N, Miura T (1998) High-performance liquid chromatographic method for rapid and highly sensitive determination of histidine using postcolumn fluorescence detection with o-phthaldialdehyde. J Chromatogr B Biomed Sci Appl 718(2):235–241. doi:10.1016/S0378-4347(98)00373-9
Hermann K, Abeck D (2001) Analysis of histidine and urocanic acid isomers by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 750(1):71–80. doi:10.1016/S0378-4347(00)00416-3
Elbaz J, Shlyahovsky B, Willner I (2008) A DNAzyme cascade for the amplified detection of Pb2+ ions or L-histidine. Chem Commun 13:1569–1571. doi:10.1039/B716774A
Scarpa M, Vianello F, Signor L, Zennaro L, Rigo A (1996) Ascorbate oxidation catalyzed by bis(histidine)copper(II). Inorg Chem 35(18):5201–5206. doi:10.1021/ic9600644
Parthasarathy S, Long F, Miller Y, Xiao Y, McElheny D, Thurber K, Ma B, Nussinov R, Ishii Y (2011) Molecular-level examination of Cu2+ binding structure for amyloid fibrils of 40-residue Alzheimer's beta by solid-state NMR spectroscopy. J Am Chem Soc 133(10):3390–3400. doi:10.1021/ja1072178
Huang Z, Du J, Zhang J, Yu X-Q, Pu L (2012) A simple and efficient fluorescent sensor for histidine. Chem Commun 48(28):3412–3414. doi:10.1039/C2CC17156B
Zhu X, Zhao T, Nie Z, Miao Z, Liu Y, Yao S (2016) Nitrogen-doped carbon nanoparticle modulated turn-on fluorescent probes for histidine detection and its imaging in living cells. Nano 8(4):2205–2211. doi:10.1039/C5NR07826A
Zhou Y, Zhou T, Zhang M, Shi G (2014) A DNA-scaffolded silver nanocluster/Cu2+ ensemble as a turn-on fluorescent probe for histidine. Analyst 139(12):3122–3126. doi:10.1039/C4AN00487F
Breaker RR, Joyce GF (1994) A DNA enzyme that cleaves RNA. Chem Biol 1(4):223–229. doi:10.1016/1074-5521(94)90014-0
Liu J, Lu Y (2004) Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J Am Chem Soc 126(39):12298–12305. doi:10.1021/ja046628h
Huang P-J J, Vazin M, Liu J (2015) Desulfurization activated Phosphorothioate DNAzyme for the detection of thallium. Anal Chem 87(20):10443–10449. doi:10.1021/acs.analchem.5b02568
Zhou W, Chen Q, Huang P-J J, Ding J, Liu J (2015) DNAzyme hybridization, cleavage, degradation, and sensing in undiluted human blood serum. Anal Chem 87(7):4001–4007. doi:10.1021/acs.analchem.5b00220
Zuo P, Yin B-C, Ye B-C (2009) DNAzyme-based microarray for highly sensitive determination of metal ions. Biosens Bioelectron 25(4):935–939. doi:10.1016/j.bi os.2009.08.024
Yin B-C, Ye B-C, Tan W, Wang H, Xie C-C (2009) An allosteric dual-DNAzyme unimolecular probe for colorimetric detection of copper (II). J Am Chem Soc 131(41):14624–14625. doi:10.1021/ja9062426
Carmi N, Balkhi SR, Breaker RR (1998) Cleaving DNA with DNA. Proc Natl Acad Sci 95(5):2233–2237
Carmi N, Breaker RR (2001) Characterization of a DNA-cleaving deoxyribozyme. Bioorg Med Chem 9(10):2589–2600. doi:10.1016/S0968-0896(01)00035-9
Liu J, Lu Y (2007) Colorimetric Cu2+ detection with a ligation DNAzyme and nanoparticles. Chem Commun 46:4872–4874. doi:10.1039/B712421J
Xu Y, Wu X-Q, Shen J-S, Zhang H-W (2015) Highly selective and sensitive recognition of histidine based on the oxidase-like activity of Cu2+ ions. RSC Adv 5(112):92114–92120. doi:10.1039/C5RA17900A
Shamsipur, M, Molaabasi, F, Shanehsaz, M, & Moosavi-Movahedi, A. A. (2015) Novel blue-emitting gold nanoclusters confined in human hemoglobin, and their use as fluorescent probes for copper (II) and histidine. Microchim Acta 182(5–6), 1131–1141. doi:10.1007/s00604-014-1428-x
Rawat, K. A, & Kailasa, S. K. (2014) Visual detection of arginine, histidine and lysine using quercetin-functionalized gold nanoparticles. Microchim Acta 181(15–16), 1917–1929. doi: 10.1007/s00604-014-1294-6
He Y, Wang X, Zhu J, Zhong S, Song G (2012) Ni2+-modified gold nanoclusters for fluorescence turn-on detection of histidine in biological fluids. Analyst 137:4005–4009. doi:10.1039/C2AN35712G
Shi F, Liu S, Su X (2014) Dopamine functionalized–CdTe quantum dots as fluorescence probes for l-histidine detection in biological fluids. Talanta 125:221–226. doi:10.1016/j.talanta.2014.02.060
He H-Z, Wang M, Chan DS-H, Leung C-H, Qiu J-W, Ma D-L (2013) A label-free G-quadruplex-based luminescent switch-on assay for the selective detection of histidine. Methods 64(3):205–211. doi:10.1016/j.ymeth.2013.05.025
Huang P, Li J, Song J, Gao N, Wu F (2016) Silver nanoparticles modified with sulfanilic acid for one-step colorimetric and visual determination of histidine in serum. Microchim Acta 183(6):1865–1872. doi:10.1007/s00604-016-1823-6
Kovach PM, Meyerhoff ME (1982) Development and application of a histidine selective biomembrane electrode. Anal Chem 54(2):217–220. doi:10.1021/ac00239a016
Acknowledgements
This project was partly financially supported by National Sciences Foundation of China (21275031), the Natural Sciences Funding of Fujian Province (2014 J06005), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT15R11), and the Foundation for Scholars of Fuzhou University (XRC-1671, XRC-17007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 163 kb)
Rights and permissions
About this article
Cite this article
Chen, Z., He, Q., Zhao, M. et al. A fluorometric histidine biosensor based on the use of a quencher-labeled Cu(II)-dependent DNAzyme. Microchim Acta 184, 4015–4020 (2017). https://doi.org/10.1007/s00604-017-2425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2425-7