Abstract
An open system with a thermal gradient is described for the optimization of polymerase chain reactions (PCR). Two thermal electric coolers were used as the heat source. The gradient is measured through encapsulated water-based beads of a temperature-dependent dye inside mineral oil, thereby forming virtual reaction chambers. Nine droplets (with typical volume of 0.7 μL) were used. Using the intrinsic fluorescence of a temperature-sensitive inert dye (sulforhodamine B), the process involves measurement of the fluorescence intensity at a known, uniform temperature together with the instrument-specific calibration constant to calculate an unknown, possibly non-uniform temperature. The results show that a nearly linear thermal gradient is obtained. This gradient function is a useful feature that can be used for optimization of a commonly used enzyme-activated reaction, viz. PCR. The emission spectra of fluorescent droplets during two-step PCR were monitored and the changes in fluorescence between 50 °C and 100 °C quantified. As the gradient feature allows for testing a range of annealing temperatures simultaneously, the optimal annealing temperature can be easily determined in a single experiment.

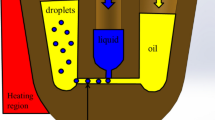
Schematic presentation of an open system with a thermal gradient designed for PCR optimization. The exact temperature-time course of the sample is monitored in real-time throughout the thermal cycling. The method ensures significant time-savings and a reduction in reagent use during optimization and standard PCR experiments.






Similar content being viewed by others
References
Kopp MU, De Mello AJ, Manz A (1998) Chemical amplification: continuous-flow PCR on a chip. Science 280:1046–1048
Ross D, Gaitan M, Locascio LE (2001) Temperature measurement in microfluidic systems using a temperature-dependent fluorescent dye. Anal Chem 73:4117–4123
Wu J, Kodzius R, Cao W, Wen W (2014) Extraction, amplification and detection of DNA in microfluidic chip-based assays. Microchim Acta 181:1611–1631
Karle M, Vashist SK, Zengerle R, von Stetten F (2016) Microfluidic solutions enabling continuous processing and monitoring of biological samples: a review. Anal Chim Acta 929:1–22
Neuzil P, Pipper J, Hsieh TM (2006) Disposable real-time microPCR device: lab-on-a-chip at a low cost. Mol BioSyst 2:292–298
Neužil P, Sun W, Karásek T, Manz A (2015) Nanoliter-sized overheated reactor. Appl Phys Lett 106:024104
Ahrberg CD, Ilic BR, Manz A, Neužil P (2016) Handheld real-time PCR device. Lab Chip 16:586–592
Wang X-d, Wolfbeis OS, Meier RJ (2013) Luminescent probes and sensors for temperature. Chem Soc Rev 42:7834–7869
Vukusic P, Hooper I (2005) Directionally controlled fluorescence emission in butterflies. Science 310:1151–1151
Hatch AC, Fisher JS, Tovar AR, Hsieh AT, Lin R, Pentoney SL, Yang DL, Lee AP (2011) 1-million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip 11:3838–3845
Ahrberg CD, Manz A, Chung BG (2016) Polymerase chain reaction in microfluidic devices. Lab Chip 16:3866–3884
Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51:263–273
Kean OW (2010) Using the gradient technology of the Mastercycler pro to generate a single universal PCR protocol for multiple primer sets. Appl Note 220
Lopez J, Prezioso V (2001) A better way to optimize: two-step gradient PCR. Eppendorf BioNews Appl Note 16:3–4
Kim H, Park N, Hahn JH (2016) Parallel-processing continuous-flow device for optimization-free polymerase chain reaction. Anal Bioanal Chem 408:6751–6758
Lagally E, Medintz I, Mathies R (2001) Single-molecule DNA amplification and analysis in an integrated microfluidic device. Anal Chem 73:565–570
Terabe S, Otsuka K, Ando T (1985) Electrokinetic chromatography with micellar solution and open-tubular capillary. Anal Chem 57:834–841
LightCycler 2.0 Instrument (2017) Product specifications. https://lifescience.roche.com/shop/en/global/products/lightcycler14301-20-instrument. Accessed 20 Feb 2017
Sanford LN, Wittwer CT (2014) Fluorescence-based temperature control for polymerase chain reaction. Anal Biochem 448:75–81
Sanford LN, Wittwer CT (2013) Monitoring temperature with fluorescence during real-time PCR and melting analysis. Anal Biochem 434:26–33
Natrajan V, Christensen K (2008) Two-color laser-induced fluorescent thermometry for microfluidic systems. Meas Sci Technol 20:015401
³Prime thermal cycler, PCR optimisation: using a gradient. http://www.techne.com/product.asp?dsl=910, Appl Note A01-001B Accessed 23 Feb 2017
Bio-Rad (2006) Real-Time PCR applications guide.
Acknowledgements
We would like to thank Christian Ahrberg (former KIST Europe employee) for help with data processing. We would like to thank Yuliya E. Silina (INM institute, Saarland University) for testing the stability of the coating method. We would like to thank Zhenjun Chang (Jiangsu University of Science and Technology) for the advice of revising the manuscript. This research was funded by the China Scholarship Council (File No. 201308330205).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 22.6 kb)
Rights and permissions
About this article
Cite this article
Li, X., Wu, W. & Manz, A. Thermal gradient for fluorometric optimization of droplet PCR in virtual reaction chambers. Microchim Acta 184, 3433–3439 (2017). https://doi.org/10.1007/s00604-017-2353-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2353-6