Abstract
The authors report on a nonenzymatic catechol sensor that is based on the immobilization of ferrocene (Fc) on graphene oxide (GO). A glassy carbon electrode (GCE) was modified with GO which then was silanized with (3-aminopropyl)trimethoxysilane. Ferrocenecarboxaldehyde was then immobilized on GO via formation of a Schiff base. The immobilization process was monitored stepwise by using FTIR spectroscopy, X-ray diffraction, cyclic voltammetry (CV) and electrochemical impedance spectroscopy. Investigation of the modified electrode by CV revealed a pair of well-defined redox peaks with anodic and cathodic peak potentials at +0.380 and +0.277 V, corresponding to the Fc/Fc+ redox couple. The Fc modified electrode exhibits excellent electrocatalytic activity towards the oxidation of catechol at a typical working voltage of +0.45 V (vs. Ag/AgCl). The response is linear in the 3 to 112 μM catechol concentration range, the detection limit is 1.1 μM, and the sensitivity is 1184.3 μA·mM−1·cm−2. The sensor is stable, reproducible and reasonably selective. It was successfully applied to the determination of catechol in spiked tap water and lake water samples.

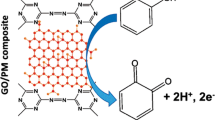
Schematic presentation of the covalent immobilization of ferrocene on graphene oxide through (3-aminopropyl)trimethoxysilane via Schiff base condensation for nonenzymatic catechol determination. The use of the electrode with covalently linked ferrocene and a graphene oxide host results in faster and enhanced amperometric response.






Similar content being viewed by others
References
Yang J, Stuart MAC, Kamperman M (2014) Jack of all trades: versatile catechol crosslinking mechanisms. Chem Soc Rev 43:8271–8298. doi:10.1039/c4cs00185k
Baco E, Hoegy F, Schalk IJ, Mislin GLA (2014) Diphenyl-benzo[1,3]dioxole-4-carboxylic acid pentafluorophenyl ester: a convenient catechol precursor in the synthesis of siderophore vectors suitable for antibiotic trojanhorse strategies. Org Biomol Chem 12:749–757. doi:10.1039/c3ob41990h
Povie G, Ford L, Pozzi D, Soulard V, Villa G, Renaud P (2016) Catechols as sources of hydrogen atoms in radical deiodination and related reactions. Angew Chem Int Ed 128:1–6. doi:10.1002/anie.201604950
Quynh BTP, Byun JY, Kim SH (2015) Non-enzymatic amperometric detection of phenol and catechol using nanoporous gold. Sens Actuators B Chem 221:191–200. doi:10.1016/j.snb.2015.06.067
Palanisamy S, Thangavelu K, Chen SM, Thirumalraj B, Liu XH (2016) Preparation and characterization of gold nanoparticles decorated on graphene oxide@polydopamine composite: application for sensitive and low potential detection of catechol. Sens Actuators B Chem 233:298–306. doi:10.1016/j.snb.2016.04.083
Sun R, Wang Y, Ni Y, Kokot S (2014) Spectrophotometric analysis of phenols, which involves a hemin-graphene hybrid nanoparticles with peroxidase-like activity. J Hazard Mater 266:60–67. doi:10.1016/j.jhazmat.2013.12.006
Proestos C, Sereli D, Komaitis M (2006) Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem 95:44–52. doi:10.1016/j.foodchem.2004.12.016
Han S, Liu B, Liu Y, Fan Z (2016) Silver nanoparticle induced chemiluminescence of the hexacyanoferrate-fluorescein system, and its application to the determination of catechol. Microchim Acta 183:917–921. doi:10.1007/s00604-015-1704-4
Liao CI, Ku KL (2012) Development of a signal-ratio-based antioxidant index for assisting the identification of polyphenolic compounds by mass spectrometry. Anal Chem 84:7440–7448. doi:10.1021/ac301283s
Sethuraman V, Muthuraja P, Raj JA, Manisankar P (2016) A highly sensitive electrochemical biosensor for catechol using conducting polymer reduced graphene oxide-metal oxide enzyme modified electrode. Biosens Bioelectron 84:112–119. doi:10.1016/j.bios.2015.12.074
Gao ZY, Gao YL, Wang E, Xu SX, Chen W (2016) Electrochemical determination of catechol based on cadmium telluride quantum dots/graphene composite film modified electrode. J Electrochem Soc 163:H528–H533. doi:10.1149/2.0681607jes
Palanisamy S, Ramaraj SK, Chen SM, Velusamy V, Yang TCK, Chen TW (2017) Voltammetric determination of catechol based on a glassy carbon electrode modified with a composite consisting of graphene oxide and polymelamine. Microchim Acta 184:1051–1057. doi:10.1007/s00604-017-2073-y
Vicentini FC, Garcia LLC, Figueiredo-Filho LCS, Janegitz BC, Fatibello-Filho O (2016) A biosensor based on gold nanoparticles, dihexadecylphosphate, and tyrosinase for the determination of catechol in natural water. Enzym Microb Technol 84:17–23. doi:10.1016/j.enzmictec.2015.12.004
Karim MN, Lee JE, Lee HJ (2014) Amperometric detection of catechol using tyrosinase modified electrodes enhanced by the layer-by-layer assembly of gold nanocubes and polyelectrolytes. Biosens Bioelectron 61:147–151. doi:10.1016/j.bios.2014.05.011
Kumar AS, Swetha P, Pillai C (2010) Enzyme-less and selective electrochemical sensing of catechol and dopamine using ferrocene bound nafion membrane modified electrode. Anal Methods 2:1962–1968. doi:10.1039/c0ay00430h
Kumar SS, Kwak K, Lee D (2011) Electrochemical sensing using quantum-sized gold nanoparticles. Anal Chem 83:3244–3247. doi:10.1021/ac200384w
Li Y, Zhai X, Wang H, Liu X, Guo L, Ji X, Wang L, Qiu H, Liu X (2015) Non-enzymatic sensing of uric acid using a carbon nanotube ionic-liquid paste electrode modified with poly(β-cyclodextrin). Microchim Acta 182:1877–1884. doi:10.1007/s00604-015-1522-8
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648. doi:10.1016/j.bios.2011.05.039
Xu J, Wang Y, Hu S (2017) Nanocomposites of graphene and graphene oxides: synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim Acta 184:1–44. doi:10.1007/s00604-016-2007-0
Cheng X, Zhang J, Chang H, Luo L, Nie F, Feng X (2016) High performance cu/Cu2O nanohybrid electrocatalyst for nonenzymatic glucose detection. J Mater Chem B 4:4652–4656. doi:10.1039/c6tb01158f
Song J, Xu L, Xing R, Li Q, Zhou C, Liu D, Song H (2014) Synthesis of au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Sci Rep 4:7515. doi:10.1038/srep07515
Song J, Xu L, Zhou C, Xing R, Dai Q, Liu D, Song H (2013) Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl Mater Interfaces 5:12928–12934. doi:10.1021/am403508f
Sudhesh P, Balamurugan T, Berchmans S (2016) Insights into ferrocene-mediated nitric oxide sensing - elucidation of mechanism and isolation of intermediate. Electrochim Acta 210:321–327. doi:10.1016/j.electacta.2016.05.153
Rabti A, Raouafi N, Merkoci A (2016) Bio(sensing) devices based on ferrocene- functionalized graphene and carbon nanotubes. Carbon 108:481–514. doi:10.1016/j.carbon.2016.07.043
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339. doi:10.1021/ja01539a017
Rajesh R, Sujanthi E, Kumar SS, Venkatesan R (2015) Designing versatile heterogeneous catalysts based on ag and au nanoparticles decorated on chitosan functionalized graphene oxide. Phys Chem Chem Phys 17:11329–11340. doi:10.1039/c5cp00682a
Zhang F, Jiang H, Li X, Wu X, Li H (2014) Amine-functionalized GO as an active and reusable acid-base bifunctional catalyst for one-pot cascade reactions. ACS Catal 4:394–401. doi:10.1021/cs400761r
Qu F, Zhang Y, Rasooly A, Yang M (2014) Electrochemical biosensing platform using hydrogel prepared from ferrocene modified amino acid as highly efficient immobilization matrix. Anal Chem 86:973–976. doi:10.1021/ac403478z
Xia N, Zhang Y, Chang K, Gai X, Jing Y, Li S, Liu L, Qu G (2015) Ferrocene-phenylalanine hydrogels for immobilization of acetylcholinesterase and detection of chlorpyrifos. J Electroanal Chem 746:68–74. doi:10.1016/j.jelechem.2015.03.030
Lu L, Huang X, Dong Y, Huang Y, Pan X, Wang X, Feng M, Luo Y, Fang D (2015) Facile method for fabrication of self-supporting nanoporous gold electrodes via cyclic voltammetry in ethylene glycol, and their application to the electrooxidative determination of catechol. Microchim Acta 182:1509–1517. doi:10.1007/s00604-015-1479-7
Mu S (2006) Catechol sensor using poly(aniline-co-o-aminophenol) as an electron transfer mediator. Biosens Bioelectron 21:1237–1243. doi:10.1016/j.bios.2005.05.007
Huang KJ, Wang L, Li J, Yu M, Liu YM (2013) Electrochemical sensing of catechol using a glassy carbon electrode modified with a composite made from silver nanoparticles, polydopamine, and graphene. Microchim Acta 180:751–757. doi:10.1007/s00604-013-0988-5
Liu L, Ma Z, Zhu X, Alshahrani LA, Tie S, Nan J (2016) A glassy carbon electrode modified with carbon nano-fragments and bismuth oxide for electrochemical analysis of trace catechol in the presence of high concentrations of hydroquinone. Microchim Acta 183:3293–3301. doi:10.1007/s00604-016-1973-6
Zhao X, He D, Wang Y, Hu Y, Fu C (2016) Au nanoparticles and graphene quantum dots co-modified glassy carbon electrode for catechol sensing. Chem Phys Lett 647:165–169. doi:10.1016/j.cplett.2016.01.019
Wang Y, Qu J, Li S, Dong Y, Qu J (2015) Simultaneous determination of hydroquinone and catechol using a glassy carbon electrode modified with gold nanoparticles, ZnS/NiS@ZnS quantum dots and L-cysteine. Microchim Acta 182:2277–2283. doi:10.1007/s00604-015-1568-7
Acknowledgements
This work was financially supported by the Science and Engineering Research Board (SERB), Government of India (Sanction No. SB/FT/CS-078/2014). The authors are thankful to SIF DST-VIT-FIST, VIT University, Vellore for providing the analytical facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 512 kb)
Rights and permissions
About this article
Cite this article
Elancheziyan, M., Manoj, D., Saravanakumar, D. et al. Amperometric sensing of catechol using a glassy carbon electrode modified with ferrocene covalently immobilized on graphene oxide. Microchim Acta 184, 2925–2932 (2017). https://doi.org/10.1007/s00604-017-2312-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2312-2