Abstract
The authors describe an electrochemical approach for the preparation of a glassy carbon electrode (GCE) modified with graphene oxide and silver nanodentrites (AgNDs). The coating was obtained by using an aqueous solution containing silver nitrate, phosphate and ammonia. The phosphate anions act as a scaffold for the improved deposition of AgNDs. The effects of deposition potential and time and concentration of electrolyte on the formation of the AgNDs were optimized. The modified GCE displays good electrocatalytic activity towards the oxidation of dissolved hydrazine. The electron transfer coefficient and diffusion coefficient are 0.60 and 4.64 × 10−5 cm2 s−1 respectively. The electrode exhibits a linear response over the 100 nM to 670 μM hydrazine concentration range and a detection limit (LOD) of 33 nM. The sensitivity of the modified electrode is 2077 μA mM−1 cm−2 at a typical working voltage of 0.1 V (vs Ag/AgCl). This LOD is much lower than that of the allowable concentration of hydrazine in drinking water as defined by the US EPA and the WHO.

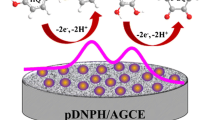
Schematic of the 2-step fabrication of a glassy carbon electrode (GCE) modified with graphene oxide (GO) and silver nanodendrites (AgND) for use in a hydrazine sensor. First, Ag3PO4 is formed by adding AgNO3 and phosphate. Secondly, the formed Ag3PO4 is converted to a colorless complex by adding ammonia and by electrolytic growth of AgND on the GO/GCE.






Similar content being viewed by others
References
Rao KM, Kumar A, Haider A, Han SS (2016) Polysaccharides based antibacterial polyelectrolyte hydrogels with silver nanoparticles. Mater Lett 184:189–192
Ensafi AA, Zandi-Atashbar N, Rezaei B, Ghiaci M, Taghizadeh M (2016) Silver nanoparticles decorated carboxylate functionalized SiO 2, new nanocomposites for non-enzymatic detection of glucose and hydrogen peroxide. Electrochim Acta 214:208–216
Jahanbakhshi M, Habibi B (2016) A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: application to electroanalytical determination of H 2 O 2 in fetal bovine serum. Biosens Bioelectron 81:143–150
Sun S, Miao H, Xue Y, Wang Q, Li S, Liu Z (2016) Oxygen reduction reaction catalysts of manganese oxide decorated by silver nanoparticles for aluminum-air batteries. Electrochim Acta 214:49–55
Noori MT, Jain SC, Ghangrekar M, Mukherjee C (2016) Biofouling inhibition and enhancing performance of microbial fuel cell using silver nano-particles as fungicide and cathode catalyst. Bioresour Technol 220:183–189
Xu S, Tang B, Zheng X, Zhou J, An J, Ning X, Xu W (2009) The facet selectivity of inorganic ions on silver nanocrystals in etching reactions. Nanotechnology 20(41):415601
Gu X, Nie C, Lai Y, Lin C (2006) Synthesis of silver nanorods and nanowires by tartrate-reduced route in aqueous solutions. Mater Chem Phys 96(2):217–222
Das R, Sarkar S (2015) X-ray diffraction analysis of synthesized silver nanohexagon for the study of their mechanical properties. Mater Chem Phys 167:97–102
Dong W, Zhang L, Li B, Wang L, Wang G, Li X, Chen B, Li C (2016) Preparation of hollow multiple-ag-nanoclustes-C-shell nanostructures and their catalytic properties. Appl Catal B Environ 180:13–19
Zhang W, Tan F, Wang W, Qiu X, Qiao X, Chen J (2012) Facile, template-free synthesis of silver nanodendrites with high catalytic activity for the reduction of p-nitrophenol. J Hazard Mater 217:36–42
Wen X, Xie Y-T, Mak WC, Cheung KY, Li X-Y, Renneberg R, Yang S (2006) Dendritic nanostructures of silver: facile synthesis, structural characterizations, and sensing applications. Langmuir 22(10):4836–4842
Gu H-X, Xue L, Zhang Y-F, Li D-W, Long Y-T (2015) Facile fabrication of a silver dendrite-integrated chip for surface-enhanced Raman scattering. ACS Appl Mater Interfaces 7(4):2931–2936
Li Y-T, Qu L-L, Li D-W, Song Q-X, Fathi F, Long Y-T (2013) Rapid and sensitive in-situ detection of polar antibiotics in water using a disposable ag–graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens Bioelectron 43:94–100
Rezaei B, Damiri S (2010) Electrodeposited silver nanodendrites electrode with strongly enhanced electrocatalytic activity. Talanta 83(1):197–204
Fang J, You H, Kong P, Yi Y, Song X, Ding B (2007) Dendritic silver nanostructure growth and evolution in replacement reaction. Cryst Growth Des 7(5):864–867
Wang X, Itoh H, Naka K, Chujo Y (2003) Tetrathiafulvalene-assisted formation of silver dendritic nanostructures in acetonitrile. Langmuir 19(15):6242–6246
Wang Y, Fan X, Sun J (2009) Hydrothermal synthesis of phosphate-mediated ZnO nanosheets. Mater Lett 63(3):350–352
Imai H, Iwai S, Yamabi S (2004) Phosphate-mediated ZnO nanosheets with a mosaic structure. Chem Lett 33(6):768–769
Yamabi S, Yahiro J, Iwai S, Imai H (2005) Formation of cellular films consisting of wurtzite-type zinc oxide nanosheets by mediation of phosphate anions. Thin Solid Films 489(1):23–30
Muthukumaran T, Philip J (2014) Enhanced thermal stability of phosphate capped magnetite nanoparticles. J Appl Phys 115(22):224304
Cai Y, Pan H, Xu X, Hu Q, Li L, Tang R (2007) Ultrasonic controlled morphology transformation of hollow calcium phosphate nanospheres: a smart and biocompatible drug release system. Chem Mater 19(13):3081–3083
Yusoff N, Rameshkumar P, Mehmood MS, Pandikumar A, Lee HW, Huang NM (2017) Ternary nanohybrid of reduced graphene oxide-nafion@ silver nanoparticles for boosting the sensor performance in non-enzymatic amperometric detection of hydrogen peroxide. Biosens Bioelectron 87:1020–1028
Kamat PV (2009) Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J Phys Chem Lett 1(2):520–527
Lightcap IV, Kosel TH, Kamat PV (2010) Anchoring semiconductor and metal nanoparticles on a two-dimensional catalyst mat. Storing and shuttling electrons with reduced graphene oxide. Nano Lett 10(2):577–583
Zhu J, Chauhan DS, Shan D, Wu X-Y, Zhang G-Y, Zhang X-J (2014) Ultrasensitive determination of hydrazine using a glassy carbon electrode modified with pyrocatechol violet electrodeposited on single walled carbon nanotubes. Microchim Acta 181(7–8):813–820
Steinhoff D, Mohr U (1988) The question of carcinogenic effects of hydrazine. Exp Pathol 33(3):133–143
Rahman MM, Khan A, Marwani HM, Asiri AM (2016) Hydrazine sensor based on silver nanoparticle-decorated polyaniline tungstophosphate nanocomposite for use in environmental remediation. Microchim Acta 183(5):1787–1796
Oh J-A, Shin H-S (2015) Simple and sensitive determination of hydrazine in drinking water by ultra-high-performance liquid chromatography–tandem mass spectrometry after derivatization with naphthalene-2, 3-dialdehyde. J Chromatogr A 1395:73–78
Pinter JS, Brown KL, DeYoung PA, Peaslee GF (2007) Amperometric detection of hydrazine by cyclic voltammetry and flow injection analysis using ruthenium modified glassy carbon electrodes. Talanta 71(3):1219–1225
Oh J-A, Park J-H, Shin H-S (2013) Sensitive determination of hydrazine in water by gas chromatography–mass spectrometry after derivatization with ortho-phthalaldehyde. Anal Chim Acta 769:79–83
Fang B, Feng Y, Liu M, Wang G, Zhang X, Wang M (2011) Electrocatalytic oxidation of hydrazine at a glassy carbon electrode modified with nickel ferrite and multi-walled carbon nanotubes. Microchim Acta 175(1–2):145
Cheng Z, Liu L, Xu S, Lu M, Wang X (2015) Temperature dependence of electrical and thermal conduction in single silver nanowire. Sci Rep 5:10718
Nie H, Horiuchi K, Yamauchi H, Masai J (1997) Local surface potential measurement of Pd/GaAs contact and anodized aluminum films using scanning probe microscopy. Nanotechnology 8(3A):A24
Fathi F, Lagugné-Labarthet F, Pedersen DB, Kraatz H-B (2012) Studies of the interaction of two organophosphonates with nanostructured silver surfaces. Analyst 137(19):4448–4453
Vasantha V, Chen S-M (2006) Synergistic effect of a catechin-immobilized poly (3, 4-ethylenedioxythiophene)-modified electrode on electrocatalysis of NADH in the presence of ascorbic acid and uric acid. Electrochim Acta 52(2):665–674
Acknowledgements
One of the authors Dr. Rajkumar Devasenathipathy would like to gratitude National Taipei University of technology for the post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 8940 kb)
Rights and permissions
About this article
Cite this article
Karuppasamy, K., Devasenathipathy, R. & Wang, SF. A glassy carbon electrode modified with graphene oxide decorated silver phosphate nanodentrites for amperometric determination of dissolved hydrazine. Microchim Acta 184, 2569–2577 (2017). https://doi.org/10.1007/s00604-017-2237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2237-9