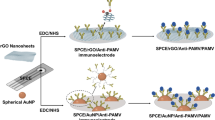
Abstract
The authors report on a robust method for the synthesis of gold nanorods (AuNRs) with tunable dimensions and longitudinal surface plasmon resonance. The method relies on seed-mediated particle growth in the presence of benzalkonium chloride (BAC) in place of the widely used surfactant cetyltrimethyl ammonium bromide (CTAB). Uniform AuNRs were obtained by particle growth in solution, and BAC is found to stabilize the AuNRs for >1 year. The SERS activity of the resulting AuNRs is essentially identical to that of CTAB-protected nanorods. The SERS activity of the BAC protected nanorods was applied to the quantitative analysis of potato virus X (PVX). The calibration plot for PVX is linear in the 10 to 750 ng⋅mL−1 concentration range, and the detection limit is 2.2 ng⋅mL−1.

SERS-active gold nanorods (AuNRs) have been prepared by using benzalkonium chloride as stabilization agent. Effects of chemical parameters on AuNRs have been explored and AuNRs were used in quantitative analysis of potato virus X (PVX).



Similar content being viewed by others
References
Schmid G (ed) (2010) Nanoparticles: from theory to application, 2nd edn. Wiley-Blackwell, Weinheim. doi:10.1002/9783527631544
Zhao P, Li N, Astruc D (2013) State of the art in gold nanoparticle synthesis. Coord Chem Rev 257(3–4):638–665. doi:10.1016/j.ccr.2012.09.002
Chen F, Wang Y, Ma J, Yang G (2014) A biocompatible synthesis of gold nanoparticles by Tris(hydroxymethyl)aminomethane. Nanoscale Res Lett 9:1–6. doi:10.1186/1556-276x-9-220
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41:2740–2779. doi:10.1039/c1cs15237h
Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ (2008) Biological applications of gold nanoparticles. Chem Soc Rev 37(9):1896–1908. doi:10.1039/b712170a
Guerrero-Martínez A, Pérez-Juste J, Carbó-Argibay E, Tardajos G, Liz-Marzán LM (2009) Gemini-surfactant-directed self-assembly of monodisperse gold nanorods into standing Superlattices. Angew Chem Int Ed 48(50):9484–9488. doi:10.1002/anie.200904118
Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Mulvaney P (2005) Gold nanorods: synthesis, characterization and applications. Coord Chem Rev 249:1870–1901. doi:10.1016/j.ccr.2005.01.030
Zia R, Schuller JA, Chandran A, Brongersma ML (2006) Plasmonics: the next chip-scale technology. Mater Today (Oxford, U K) 9:20–27. doi:10.1016/s1369-7021(06)71572-3
Yu C, Varghese L, Irudayaraj J (2007) Surface modification of cetyltrimethylammonium bromide-capped gold nanorods to make molecular probes. Langmuir 23(17):9114–9119. doi:10.1021/la701111e
Ye X, Gao Y, Chen J, Reifsnyder DC, Zheng C, Murray CB (2013) Seeded growth of monodisperse gold nanorods using bromide-free surfactant mixtures. Nano Lett 13(5):2163–2171. doi:10.1021/nl400653s
Schacter D (2013) The source of toxicity in ctab and ctab-stabilized gold nanorods. Master of Science Thesis, The State University of New Jersey. doi:10.7282/T3X63KMS
Johnson CJ, Dujardin E, Davis SA, Murphy CJ, Mann S (2002) Growth and form of gold nanorods prepared by seed-mediated, surfactant-directed synthesis. J Mater Chem 12(6):1765–1770. doi:10.1039/b200953f
Liu G-SP (2005) Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J Phys Chem B 109(47):22192–22200. doi:10.1021/jp054808n
Orendorff CJ, Murphy CJ (2006) Quantitation of metal content in the silver-assisted growth of gold nanorods. J Phys Chem B 110(9):3990–3994. doi:10.1021/jp0570972
Choi B-S, Iqbal M, Lee T, Kim YH, Tae G (2008) Removal of cetyltrimethylammonium bromide to enhance the biocompatibility of Au nanorods synthesized by a modified seed mediated growth process. J Nanosci Nanotechnol 8:4670–4674. doi:10.1166/jnn.2008.IC18
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1(3):325–327. doi:10.1002/smll.200400093
Koyama K, Shimazu Y (2005) Benzalkonium chlorides. Springer GmbH, pp 407–413. doi:10.1007/3-540-27579-7_45
Freeman PD, Kahook MY (2009) Preservatives in topical ophthalmic medications: historical and clinical perspectives. Expert Review of Ophthalmology 4(1):59–64. doi:10.1586/17469899.4.1.59
Pisella PJ, Fillacier K, Elena PP, Debbasch C, Baudouin C (2000) Comparison of the effects of preserved and unpreserved formulations of Timolol on the ocular surface of albino rabbits. Ophthalmic Res 32(1):3–8
Törnblom M, Henriksson U (1997) Effect of Solubilization of aliphatic hydrocarbons on size and shape of Rodlike C16TABr micelles studied by 2H NMR relaxation. J Phys Chem B 101(31):6028–6035. doi:10.1021/jp970899f
Zhu Y, Free ML (2015) Evaluation of ion effects on surfactant aggregation from improved molecular thermodynamic modeling. Ind Eng Chem Res 54:9052–9056. doi:10.1021/acs.iecr.5b02103
Grzelczak M, Perez-Juste J, Mulvaney P, Liz-Marzan LM (2008) Shape control in gold nanoparticle synthesis. Chem Soc Rev 37(9):1783–1791. doi:10.1039/b711490g
Schluecker S (2011) SERS microscopy: nanoparticle probes and biomedical applications. In: Wiley-VCH Verlag GmbH & Co. KGaA, pp 263–283. doi:10.1002/9783527632756.ch12
Wang Y, Wang E (2010) Nanoparticle SERS substrates. In: Surface enhanced Raman spectroscopy. Wiley-VCH Verlag GmbH & Co. KGaA, pp 39–69. doi:10.1002/9783527632756.ch2
Yang Y, Liu J, Fu Z-W, Qin D (2014) Galvanic replacement-free deposition of Au on Ag for Core–Shell Nanocubes with enhanced chemical stability and SERS activity. J Am Chem Soc 136(23):8153–8156. doi:10.1021/ja502472x
Rycenga M, Xia X, Moran CH, Zhou F, Qin D, Li Z-Y, Xia Y (2011) Generation of hot spots with silver Nanocubes for single-molecule detection by surface-enhanced Raman scattering. Angew Chem Int Ed 50(24):5473–5477. doi:10.1002/anie.201101632
Guven B, Basaran-Akgul N, Temur E, Tamer U, Boyac IH (2011) SERS-based sandwich immunoassay using antibody coated magnetic nanoparticles for Escherichia coli enumeration. Analyst 136(4):740–748. doi:10.1039/c0an00473a
Ulman A (1996) Formation and structure of self-assembled monolayers. Chem Rev (Washington, DC) 96:1533–1554. doi:10.1021/cr9502357
Batten JS, Yoshinari S, Hemenway C (2003) Potato virus X: a model system for virus replication, movement and gene expression. Mol Plant Pathol 4(2):125–131. doi:10.1046/j.1364-3703.2003.00156.x
Tamer U, Guendogdu Y, Boyaci IH, Pekmez K (2010) Synthesis of magnetic core-shell Fe3O4-Au nanoparticle for biomolecule immobilization and detection. J Nanopart Res 12:1187–1196. doi:10.1007/s11051-009-9749-0
Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed 48(1):60–103. doi:10.1002/anie.200802248
Alonso-González P, Albella P, Schnell M, Chen J, Huth F, García-Etxarri A, Casanova F, Golmar F, Arzubiaga L, Hueso LE, Aizpurua J, Hillenbrand R (2012) Resolving the electromagnetic mechanism of surface-enhanced light scattering at single hot spots. Nat Commun 3:684. doi:10.1038/ncomms1674l
Weilbach A, Sander E (2000) Quantitative detection of potato viruses X and Y (PVX, PVY) with antibodies raised in chicken egg yolk (IgY) by ELISA variants. Journal of Plant Diseases and Protection 107(3):318–328
Le Ru EC, Blackie E, Meyer M, Etchegoin PG (2007) Surface enhanced Raman scattering enhancement factors: a comprehensive study. J Phys Chem C 111(37):13794–13803. doi:10.1021/jp0687908
Ma P, Liang F, Wang D, Yang Q, Ding Y, Yu Y, Gao D, Song D, Wang X (2015) Ultrasensitive determination of formaldehyde in environmental waters and food samples after derivatization and using silver nanoparticle assisted SERS. Microchim Acta 182(3):863–869. doi:10.1007/s00604-014-1400-9
Bu Y, Lee SW (2015) Flower-like gold nanostructures electrodeposited on indium tin oxide (ITO) glass as a SERS-active substrate for sensing dopamine. Microchim Acta 182(7):1313–1321. doi:10.1007/s00604-015-1453-4
Pan Y, Guo X, Zhu J, Wang X, Zhang H, Kang Y, Wu T, Du Y (2015) A new SERS substrate based on silver nanoparticle functionalized polymethacrylate monoliths in a capillary, and it application to the trace determination of pesticides. Microchim Acta 182(9):1775–1782. doi:10.1007/s00604-015-1514-8
Bamrungsap S, Treetong A, Apiwat C, Wuttikhun T, Dharakul T (2016) SERS-fluorescence dual mode nanotags for cervical cancer detection using aptamers conjugated to gold-silver nanorods. Microchim Acta 183(1):249–256. doi:10.1007/s00604-015-1639-9
Villa JEL, Santos DP, Poppi RJ (2016) Fabrication of gold nanoparticle-coated paper and its use as a sensitive substrate for quantitative SERS analysis. Microchim Acta 183(10):2745–2752. doi:10.1007/s00604-016-1918-0
Vassalini I, Rotunno E, Lazzarini L, Alessandri I (2015) “Stainless” gold nanorods: preserving shape, optical properties, and SERS activity in oxidative environment. ACS Appl Mater Interfaces 7(33):18794–18802. doi:10.1021/acsami.5b07175
Zhang Q, Han L, Jing H, Blom DA, Lin Y, Xin HL, Wang H (2016) Facet control of gold nanorods. ACS Nano 10(2):2960–2974. doi:10.1021/acsnano.6b00258
Acknowledgements
The authors acknowledge COST CA15114 -TUBITAK Project no: 114Z783 for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interest.
Electronic supplementary material
ESM 1
(DOCX 1738 kb)
Rights and permissions
About this article
Cite this article
Caglayan, M.G., Kasap, E., Cetin, D. et al. Fabrication of SERS active gold nanorods using benzalkonium chloride, and their application to an immunoassay for potato virus X. Microchim Acta 184, 1059–1067 (2017). https://doi.org/10.1007/s00604-017-2102-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2102-x