Abstract
An agent-free microwave-assisted method was developed for the preparation of a reduced graphene oxide/Fe3O4@gold nanocomposite. This material was used as an adsorbent for magnetic solid-phase extraction of organochlorine pesticides (OCPs) from water samples. The nanocomposite was characterized by transmission electron microscopy, scanning electron microscopy, Fourier transform infrared spectroscopy and energy-dispersive X-ray spectroscopy. The effects of sample volume, amount of sorbent, eluent volume, extraction and desorption time, and the effect of salt on the extraction efficiency were optimized. The linear response range of GC analysis extends from 0.05 to 500 μg L−1 of OCPs, the limits of detection range from 0.4 to 4.1 ng L−1, relative standard deviations from 1.7 to 7.3 %, and recoveries (from spiked seawater samples) from 69 to 114 %.

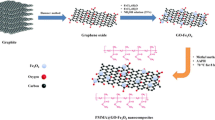
Oxygen functional groups in graphene oxide can bind to magnetic Fe3O4 nanoparticles through chemical bonds, and a magnetic reduced graphene oxide (RGO) is thus obtained. The interaction between magnetic RGO and HAuCl4 led to dispersion of the gold nanoparticles on the substrate. π-interaction of RGO and AuNPs with aromatic rings made this sorbent effective for extraction of organochlorine pesticides.






Similar content being viewed by others
References
Mehdinia A, Khani H, Mozaffari S (2014) Fibers coated with a graphene-polyaniline nanocomposite for the headspace solid-phase microextraction of organochlorine pesticides from seawater samples. Microchim Acta 181:89–95. doi:10.1007/s00604-013-1071-y
Jiang X, Huang K, Deng D, Xia H, Hou X, Zheng C (2012) Nanomaterials in analytical atomic spectrometry. TrAC Trends Anal Chem 39:38–59. doi:10.1016/j.trac.2012.06.002
Luo YB, Shi ZG, GAO QA, Feng YQ (2011) Magnetic retrieval of graphene: extraction of sulfonamide antibiotics from environmental water samples. J. Chromatogr. A 1218:1353–1358. doi:10.1016/j.chroma.2011.01.022
Han Q, Wang ZH, Xia JF, Chen S, Zhang XQ, Ding MY (2012) Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 101:388–395. doi:10.1016/j.talanta.2012.09.046
Yan S, Qi TT, Chen DW, Li Z, Li XJ, Pan SY (2014) Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J. Chromatogr. A 1347:30–38. doi:10.1016/j.chroma.2014.04.073
Wu J, Xiao D, Zhao H, He H, Peng J, Wang C, Zhang C, He J (2015) A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchim Acta 182:2299–2306. doi:10.1007/s00604-015-1575-8
Liu Q, Shi J, Wang T, Guo F, Liu L, Jiang G (2012) Hemimicelles/admicelles supported on magnetic graphene sheets for enhanced magnetic solid-phase extraction. J Chromatogr A 1257:1–8. doi:10.1016/j.chroma.2012.08.028
Han Q, Wang Z, Xia J, Chen S, Zhang X, Ding M (2012) Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 101:388–395. doi:10.1016/j.talanta.2012.09.046
Junyong C, Yongmei H, Yan L, JiaJia G (2013) Magnetic graphene oxides highly effective adsorbents for rapid removal of a cationic dye rhodamine B from aqueous solutions. RSC adv 3:7254–7258. doi:10.1039/c3ra22599b
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. doi:10.1021/cr030698+
Liu FK (2009) Analysis and applications of nanoparticles in the separation sciences: a case of gold nanoparticles. J Chromatogr A 1216:9034–9047. doi:10.1016/j.chroma.2009.07.026
Cho SJ, Kauzlarich SM, Olamit J, Liu K, Grandjean F, Rebbouh L, Long GJ (2004) Characterization and magnetic properties of core/shell structured Fe/Au nanoparticles J. Appl Phys 95:6804–6806. doi:10.1063/1.1676033
Stoeva SI, Huo F, Lee JS, Mirkin CA (2005) Three-layer composite magnetic nanoparticle probes for DNA. J Am Chem Soc 127:15362–15363. doi:10.1021/ja055056d
Deng X, Lin K, Chen X, Guo Q, Yao P (2013) Preparation of magnetic Fe3O4/Au composites for extraction of benzo[a]pyrene from aqueous solution. Chem Eng J 225:656–663. doi:10.1016/j.cej.2013.04.004
Stoeva SI, Huo FW, Lee JS, Mirkin CA (2005) Three-layer composite magnetic nanoparticle probes for DNA. J Am Chem Soc 127:15362–15363. doi:10.1021/ja055056d
Bao J, Chen W, Liu T, Zhu Y, Jin P, Wang L, Liu J, Wei Y, Li Y (2007) Bifunctional Au-Fe3O4 nanoparticles for protein separation. ACS Nano 1:293–298. doi:10.1021/nn700189h
Xuan S, Wang Y, Yu JC, Leung KC (2009) Preparation, characterization, and catalytic activity of core/shell Fe3O4@polyaniline@Au nanocomposites. Langmuir 25:11835–11843. doi:10.1021/la901462t
Zhao X, Cai Y, Wang T, Shi Y, Jiang G (2008) Preparation of alkanethiolate-functionalized core/shell Fe3O4@Au nanoparticles and its interaction with several typical target molecules. Anal Chem 80:9091–9096. doi:10.1021/ac801581m
Mehdinia A, Khojasteh E, Baradaran Kayyal T, Jabbari A (2014) Magnetic solid phase extraction using gold immobilized magnetic mesoporous silica nanoparticles coupled with dispersive liquid–liquid microextraction for determination of polycyclic aromatic hydrocarbons. J. Chromatogr. A 1364:20–27. doi:10.1016/j.chroma.2014.08.063
Ulman A (1991) An introduction to ultrathin organic films. Academic Press, Boston
Cortada C, Vidal L, Pastor R, Santiago N, Canals A (2009) Determination of organochlorine pesticides in water samples by dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. Anal Chim Acta 649:218–221. doi:10.1016/j.aca.2009.07.041
Thomann RV (1989) Bioaccumulation model of organic chemical distribution in aquatic food chains. Environ Sci Technol 23:699–707. doi:10.1021/es00064a008
Nakata H, Kawazoe M, Arizono K, Abe S, Kitano T, Shimada H, Li W, Ding X (2002) Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissues from China: status of contamination, historical trend, and human dietary exposure. Arch Environ Contam Toxicol 43:473–480. doi:10.1007/s00244-002-1254-8
Vallack HW, Bakker DJ, Brandt I, Lundén EB, Brouwer A, Bull KR, Gough C, Guardans R, Holoubek I, Jansson B, Koch R, Kuylenstierna J, Lecloux A, Mackay D, McCutcheon P, Mocarelli P, Taalman RDF (1998) Controlling persistent organic pollutants–what next? Environ Toxicol Pharmacol 6:143–175. doi:10.1016/S1382-6689(98)00036-2
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. doi:10.1021/nn1006368
Kassaee MZ, Motamedi E, Majdi M (2011) Magnetic Fe3O4-graphene oxide/polystyrene: fabrication and characterization of a promising nanocomposite. Chem Eng J 172:540–549. doi:10.1016/j.cej.2011.05.093
Song J, Xu L, Xing R, Li Q, Zhou C, Liu D, Song H (2014) Synthesis of Au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Sci Report 4:7515–7521. doi:10.1038/srep07515
Liu H, Su X, Duan C, Dong X, Zhou S, Zhu Z (2014) Microwave-assisted hydrothermal synthesis of Au NPs–graphene composites for H2O2 detection. J Electroanal Chem 731:36–42. doi:10.1016/j.jelechem.2014.08.013
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites:one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4:550–562. doi:10.1007/s12274-011-0111-3
Verdonck L, Hoste S, Roelandt FF, Van der Kelen GP (1982) Normal coordinate analysis of α-FeOOH - a molecular approach. J Mol Struct 79:273–279. doi:10.1016/0022-2860(82)85065-5
Ratola N, Santos L, Herbert P, Alves A (2006) Uncertainty associated to the analysis of organochlorine pesticides in water by solid-phase microextraction/gas chromatography–electron capture detection—evaluation using two different approaches. Anal Chim Acta 573–574:202–208. doi:10.1016/j.aca.2006.03.065
Zambonin CG, Aresta A, Nilsson T (2002) Analysis of organochlorine pesticides by solid-phase microextraction followed by gas chromatography-mass spectrometry. Int J Environ Anal Chem 82:651–657. doi:10.1080/0306731021000075375
Leong MI, Huang SD (2008) Dispersive liquid–liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J Chromatogr A 1211:8–12. doi:10.1016/j.chroma.2008.09.111
Jiang X, Wu M, Wu W, Cheng J, Zhou H, Cheng M (2014) A novel dispersive micro-solid phase extraction method combined with gas chromatography for analysis of organochlorine pesticides in aqueous samples. Anal Methods 6:9712–9717. doi:10.1039/C4AY02302A
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOC 472 kb)
Rights and permissions
About this article
Cite this article
Mehdinia, A., Rouhani, S. & Mozaffari, S. Microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles, and its application to solid-phase extraction of organochlorine pesticides. Microchim Acta 183, 1177–1185 (2016). https://doi.org/10.1007/s00604-015-1691-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1691-5