Abstract
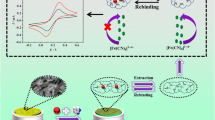
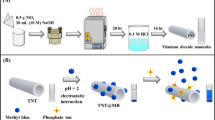
A biomimetic ion-responsive single nanopore sensor was constructed by immobilizing zirconium(IV) ion on a film of poly(ethylene terephthalate) whose surface was modified with polyethyleneimine (PEI) to form nanopores. PEI is an excellent metal-chelating ligand that was initially modified on the surface of the membrane through amidation. The Zr-chelated nanopore responds to inorganic phosphate by a change in the ion current–voltage plot. This is due to a reversal of the charge from positive to negative after binding of the phosphate to the tip of the ion channel. Inorganic phosphate can be quantified by measurement of the extent of rectification. The limit of detection of the method is 118 nM, and response is linear in the 0 to 40 μM phosphate concentration range. The formation of the Zr(IV)/phosphate system on the surface was corroborated by X-ray photoelectron spectroscopy and current–voltage characterization. The single synthetic nanopore display excellent selectivity, high sensitivity and a wide response range for phosphate. The accuracy of the method and applicability of the nanopore-based detection scheme was proven by the successful determination of inorganic phosphate in (spiked) lake water and a cola drink.

The Zr-chelated nanopore responds to inorganic phosphate by a change in the ion current–voltage plot due to a reversal of the charge from positive to negative after binding of the phosphate to the tip of the ion channel. Inorganic phosphate can be quantified by measurement of the extent of rectification. Nanopore-based assay can successful determine inorganic phosphate in (spiked) lake water and a cola drink.







Similar content being viewed by others
References
Lawal AT, Adeloju SB (2013) Progress and recent advances in phosphate sensors: a review. Talanta 114:191–203
Lurling M, Waajen G, van Oosterhout F (2014) Humic substances interfere with phosphate removal by lanthanum modified clay in controlling eutrophication. Water Res 54:78–88
Cho ES, Ahn KH, Molof AH (2004) Improvement of denitrification by denitrifying phosphorus removing bacteria using sequentially combined carbon. Water Sci Technol 50:33–40
Halliwell DJ, McKelvie ID, Hart BT, Dunhill RH (1996) Separation and detection of condensed phosphates in waste waters by ion chromatography coupled with flow injection. Analyst 121:1089–1093
Liu JF, Jiang GB (2000) Selective determination of orthophosphate and total inorganic phosphates in detergents by flow injection photometric method. Talanta 52:211–216
Pena-Pereira F, Cabaleiro N, de la Calle I, Costas M, Gil S, Lavilla I, Bendicho C (2011) Directly suspended droplet microextraction in combination with microvolume UV–vis spectrophotometry for determination of phosphate. Talanta 85:1100–1104
Spangler C, Schaeferling M, Wolfbeis OS (2008) Fluorescent probes for microdetermination of inorganic phosphates and biophosphates. Microchim Acta 161:1–39
Ding S-N, Li C-M, Gao B-H, Kargbo O, Wan N, Chen X, Zhou C (2014) Probing phosphate ion via the europium (III)-modulated fluorescence of gold nanoclusters. Microchim Acta 181:1957–1963
Berchmans S, Issa TB, Singh P (2012) Determination of inorganic phosphate by electroanalytical methods: a review. Anal Chim Acta 729:7–20
Ying YL, Li DW, Li Y, Lee JS, Long YT (2011) Enhanced translocation of poly(dt)(45) through an alpha-hemolysin nanopore by binding with antibody. Chem Commun 47:5690–5692
Wang HY, Ying YL, Li Y, Kraatz HB, Long YT (2011) Nanopore analysis of beta-amyloid peptide aggregation transition induced by small molecules. Anal Chem 83:1746–1752
Ying YL, Wang HY, Sutherland TC, Long YT (2011) Monitoring of an ATP-binding aptamer and its conformational changes using an alpha-hemolysin nanopore. Small 7:87–94
Ying YL, Zhang JJ, Meng FN, Cao C, Yao XY, Willner I, Tian H, Long YT (2013) A stimuli-responsive nanopore based on a photoresponsive host-guest system. Sci Rep 3:1662
Han C, Su H, Sun Z, Wen L, Tian D, Xu K, Hu J, Wang A, Li H, Jiang L (2013) Biomimetic ion nanochannels as a highly selective sequential sensor for zinc ions followed by phosphate anions. Chem-Eur J 19:9388–9395
Zhang S, Bao A, Sun T, Wang E, Wang J (2015) PEI/Zr4 + −coated nanopore for selective and sensitive detection of ATP in combination with single-walled carbon nanotubes. Biosen Bioelectron 63:287–293
Hou GL, Peng ZJ, Tian Y, Zhang HC, Jiang L (2013) Applications of polymer single nanochannels in biosensors. Chin Sci Bull 58:1473–1482
Zhang S, Sun T, Wang E, Wang J (2014) Investigation of self-assembled protein dimers through an artificial ion channel for DNA sensing. Chin Sci Bull 59:4946–4952
Zhang H, Tian Y, Jiang L (2013) From symmetric to asymmetric design of bio-inspired smart single nanochannels. Chem Commun 49:10048–10063
Zhai Q, Zhang S, Jiang H, Wei Q, Wang E, Wang J (2014) Biomimetic nanopore for sensitive and selective detection of Hg(II) in conjunction with single-walled carbon nanotubes. J Mater Chem B 2:6371–6377
Hou X, Guo W, Xia F, Nie FQ, Dong H, Tian Y, Wen LP, Wang L, Cao LX, Yang Y, Xue JM, Song YL, Wang YG, Liu DS, Jiang L (2009) A biomimetic potassium responsive nanochannel: G-quadruplex DNA conformational switching in a synthetic nanopore. J Am Chem Soc 131:7800–7805
Guo W, Tian Y, Jiang L (2013) Asymmetric Ion transport through Ion-channel-mimetic solid-state nanopores. Acc Chem Res 46:2834–2846
Guo W, Xia H, Xia F, Hou X, Cao L, Wang L, Xue J, Zhang G, Song Y, Zhu D, Wang Y, Jiang L (2010) Current rectification in temperature-responsive single nanopores. ChemPhysChem 11(4):859–864
Guo Z, Wang J, Ren J, Wang E (2011) pH-reversed ionic current rectification displayed by conically shaped nanochannel without any modification. Nanoscale 3:3767–3773
Xia F, Guo W, Mao YD, Hou X, Xue JM, Xia HW, Wang L, Song YL, Ji H, Qi OY, Wang YG, Jiang L (2008) Gating of single synthetic nanopores by proton-driven DNA molecular motors. J Am Chem Soc 130:8345–8350
Zhang X, Zhu Z, Sun C, Zhu F, Luo Z, Yan J, Mao B (2009) Colloidal lithography-based fabrication of suspended nanoporous silicon nitride membranes. Microchim Acta 167:135–140
Liu S, Dong Y, Zhao W, Xie X, Ji T, Yin X, Liu Y, Liang Z, Momotenko D, Liang D, Girault HH, Shao Y (2012) Studies of ionic current rectification using polyethyleneimines coated glass nanopipettes. Anal Chem 84:5565–5573
Tian Y, Hou X, Wen LP, Guo W, Song YL, Sun HZ, Wang YG, Jiang L, Zhu DB (2010) A biomimetic zinc activated ion channel. Chem Commun 46:1682–1684
Tian Y, Zhang Z, Wen LP, Ma J, Zhang YQ, Liu WD, Zhai J, Jiang L (2013) A biomimetic mercury(II)-gated single nanochannel. Chem Commun 49:10679–10681
Jiang Y, Liu N, Guo W, Xia F, Jiang L (2012) Highly-efficient gating of solid-state nanochannels by DNA supersandwich structure containing ATP aptamers: a nanofluidic IMPLICATION logic device. J Am Chem Soc 134:15395–15401
Sathe C, Zou XQ, Leburton JP, Schulten K (2011) Computational investigation of DNA detection using graphene nanopores. ACS Nano 5:8842–8851
Ling Y, Li JX, Qu F, Li NB, Luo HQ (2014) Rapid fluorescence assay for Sudan dyes using polyethyleneimine-coated copper nanoclusters. Microchim Acta 181:1069–1075
Moss RE, Perez-Roa RE, Anderson MA (2013) Electrochemical response of titania, zirconia, and alumina electrodes to phosphate adsorption. Electrochim Acta 104:314–321
Wang J, Martin CR (2008) A new drug-sensing paradigm based on ion-current rectification in a conically shaped nanopore. Nanomedicine 3:13–20
Qi W, Zhao J, Zhang W, Liu Z, Xu M, Anjum S, Majeed S, Xu G (2013) Visual and surface plasmon resonance sensor for zirconium based on zirconium-induced aggregation of adenosine triphosphate-stabilized gold nanoparticles. Anal Chim Acta 787:126–131
Guo Z, Wang J, Wang E (2012) Selective discrimination of small hydrophobic biomolecules based on ion-current rectification in conically shaped nanochannel. Talanta 89:253–257
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NO. 21275137) and the Fundamental Research Funds of Shandong University (NO. 2015TB005). The authors also thank GSI for providing track-etched membranes.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 399 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Sun, T. & Wang, J. Biomimetic phosphate assay based on nanopores obtained by immobilization of zirconium(IV) on a film of polyethyleneimine. Microchim Acta 182, 1387–1393 (2015). https://doi.org/10.1007/s00604-015-1459-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1459-y