Abstract
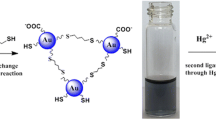
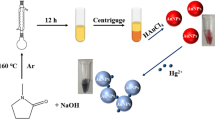
We have developed a simple method for the highly selective colorimetric detection of dissolved mercury(II) ions via direct formation of gold nanoparticles (AuNPs). The dithia-diaza ligand 2-[3-(2-amino-ethylsulfanyl)-propylsulfanyl]-ethylamine (AEPE) was used as a stabilizer to protect AuNPs from aggregation and to impart highly selective recognition of Hg(II) ion over other metal ions. A solution of Au(III) ion is directly reduced by sodium borohydride in the presence of AEPE and the detergent Triton X-100. This results in the formation of AEPE-AuNPs and a red coloration of the solution. On the other hand, in the presence of Hg(II), the solution turns blue within a few seconds after the addition of borohydride. This can be detected spectrophotometrically or even visually. The method was successfully applied to quantify Hg(II) levels in water sample, with a minimum detectable concentration as low as 35 nM.

A rapid colorimetric method for Hg2+ detection based on the reduction of Au3+ to gold nanoparticles in the presence of dithia-diaza (2S-2N) ligand was developed. The colors of the solutions without and with Hg2+ were red and blue, respectively.




Similar content being viewed by others
References
Yang H, Zhou Z, Li F, Yi T, Huang C (2007) New Hg2+ and Ag+ selective colorimetric sensor based on thiourea subunits. Inorg Chem Commun 10:1136–1139
Diez-Gil C, Caballero A, Ratera I, Tarraga A, Molina P, Veciana J (2007) Naked-eye and selective detection of mercury(II) ions in mixed aqueous media using a cellulose-based support. Sensors 7:3481–3488
Tan J, Yan XP (2008) 2,1,3-Benzoxadiazole-based selective chromogenic chemosensor for rapid naked-eye detection of Hg2+ and Cu2+. Talanta 76:9–14
Leng B, Zou L, Jiang J, Tian H (2009) Colorimetric detection of mercuric ion (Hg2+) in aqueous media using chemodosimeter-functionalized gold nanoparticles. Sensor Actuat B 140:162–169
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105:1547–1562
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Ma M, Wang J, Zheng X (2011) Enhancement of the colorimetric sensitivity of gold nanoparticles with triethanolamine to minimize interparticle repulsion. Microchim Acta 172:155–162
Huang CC, Chang HT (2007) Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem Commun 12:1215–1217
Xu Q, Du S, Jin G, Li H, Hu X (2011) Determination of acetamiprid by a colorimetric method based on the aggregation of gold nanoparticles. Microchim Acta 173:323–329
Darbha GK, Singh AK, Rai US, Yu E, Yu H, Chandra RP (2008) Selective detection of mercury(II) ion using nonlinear optical properties of gold nanoparticles. J Am Chem Soc 130:8038–8043
Yu CJ, Tseng WL (2008) Colorimetric detection of mercury(II) in a high-salinity solution using gold nanoparticles capped with 3-mercaptopropionate acid and adenosine monophosphate. Langmuir 24:12717–12722
Hung YL, Hsiung TM, Chen YY, Huang YF, Huang CC (2010) Colorimetric detection of heavy metal ions using label-free gold nanoparticles and alkanethiols. J Phys Chem C 114:16329–16334
Liu D, Qu W, Chen W, Zhang W, Wang Z, Jiang X (2010) Highly sensitive, colorimetric detection of mercury(II) in aqueous media by quaternary ammonium group-capped gold nanoparticles at room temperature. Anal Chem 82:9606–9610
Hirayama T, Taki M, Kashiwagi Y, Nakamoto M, Kunishita A, Itoh S, Yamamoto Y (2008) Colorimetric response to mercury-induced abstraction of triethylene glycol ligands from a gold nanoparticle surface. Dalton Trans 35:4705–4707
Si S, Kotal A, Mandal TK (2007) One-dimensional assembly of peptide-functionalized gold nanoparticles: an approach toward mercury ion sensing. J Phys Chem C 111:1248–1255
Kim YR, Mahajan RK, Kim JS, Kim H (2010) Highly sensitive gold nanoparticle-based colorimetric sensing of mercury(II) through simple ligand exchange reaction in aqueous media. ACS Appl Mater Interfaces 2:292–295
Xu X, Wang J, Jiao K, Yang X (2009) Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens Bioelectron 24:3153–3158
Li T, Dong S, Wang E (2009) Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+-modulated G-quadruplex-based DNAzymes. Anal Chem 81:2144–2149
Liu ZD, Li YF, Ling J, Huang CZ (2009) A localized surface plasmon resonance light-scattering assay of mercury (II) on the basis of Hg2+-DNA complex induced aggregation of gold nanoparticles. Environ Sci Technol 43:5022–5027
Liu CW, Huang CC, Chang HT (2009) Highly selective DNA-based sensor for lead(II) and mercury(II) ions. Anal Chem 81:2383–2387
Lin CY, Yu CJ, Lin YH, Tseng WL (2010) Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal Chem 82:6830–6837
Aragay G, Pons J, Ros J, Merkoci A (2010) Aminopyrazole-based ligand induces gold nanoparticle formation and remains available for heavy metal ions sensing. A simple mix and detect approach. Langmuir 26:10165–10170
Fan A, Ling Y, Lau C, Lu J (2010) Direct colorimetric visualization of mercury (Hg2+) based on the formation of gold nanoparticles. Talanta 82:687–692
Puanngam M, Unob F (2008) Preparation and use of chemically modified MCM-41 and silica gel as selective adsorbents for Hg(II) ions. J Hazard Mater 154:578–587
Phothitontimongkol T, Siebers N, Sukpirom N, Unob F (2009) Preparation and characterization of novel organo-clay minerals for Hg(II) ions adsorption from aqueous solution. Appl Clay Sci 43:343–349
Wanichacheva N, Kamkaew A, Watpathomsub S, Lee VS, Grudpan K (2010) 2-[3-(2-Aminoethylsulfanyl)propylsulfanyl]ethanamine bearing dansyl subunits: an efficient, simple, and rapid fluorometric sensor for the detection of mercury(II) ions. Chem Lett 39:1099–1101
Choudhury SB, Ray D, Chakravorty A (1991) Sulfur-ligated nickel oxidation states. Chemistry of family of NizS2N4 (z = +2, +3, +4) complexes incorporating hexadentate thioether-imine-oxime binding. Inorg Chem 30:4354–4360
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862
Nguyen DT, Kim DJ, So MG, Kim KS (2010) Experimental measurements of gold nanoparticle nucleation and growth by citrate reduction of HAuCl4. Adv Powder Technol 21:111–118
Zhang Z, Wu Y (2010) Investigation of the NaBH4-induced aggregation of Au nanoparticles. Langmuir 26:9214–9223
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539
Thomas KG, Zajicek J, Kamat PV (2002) Surface binding properties of tetraoctylammonium bromide-capped gold nanoparticles. Langmuir 18:3722–3727
Rex M, Hernandez FE, Campiglia AD (2006) Pushing the limits of mercury sensors with gold nanorods. Anal Chem 78:445–451
Acknowledgements
This study was carried out in the Environmental Analysis Research Unit (EARU) financially supported by Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (GRU 53-005-23-003 and FW002A), the program Strategic Scholarships for Frontier Research Network for the Ph.D. Program Thai Doctoral degree and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (FW652I). Dr. Robert Butcher, Publication Counseling Unit, Faculty of Science, Chulalongkorn University is also acknowledged for English corrections and suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 211 kb)
Rights and permissions
About this article
Cite this article
Chansuvarn, W., Imyim, A. Visual and colorimetric detection of mercury(II) ion using gold nanoparticles stabilized with a dithia-diaza ligand. Microchim Acta 176, 57–64 (2012). https://doi.org/10.1007/s00604-011-0691-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0691-3