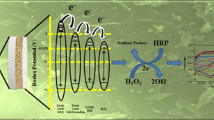
Abstract
An electrochemical sensor was developed for determination of hydrogen peroxide (HP) based on a carbon ceramic electrode modified with iron pentacyanonitrosylferrate (FePCNF). The surface of an iron-doped CCE was derivatized in a solution of PCNF by cycling the electrode potential between −0.2 and +1.3 V for about 60 times. The morphology and the composition of the resulting electrode were characterized by scanning electron microscopy and Fourier transform infrared techniques. The electrode displayed excellent response to the electro-oxidation of HP which is linearly related to its concentration in the range from 0.5 μM to 1300 μM. The detection limit is 0.4 μM, and the sensitivity is 849 A M −1 cm −2. The modified electrode was used to determination of HP in hair coloring creams as real samples.







Similar content being viewed by others
References
Wang CL, Mulchandani A (1995) Ferrocene-conjugated polyaniline-modified enzyme electrodes for determination of peroxides in organic media. Anal Chem 67:1109
Matsubara C, Kawamoto N, Takamura K (1992) Oxo [5, 10, 15, 20-tetra(4-pyridyl)porphyrinato] titanium(IV): an ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst 117:1781
Hanaoka S, Lin JM, Yamada M (2001) Chemiluminescent flow sensor for H2O2 based on the decomposition of H2O2 catalyzed by cobalt(II)-ethanolamine complex immobilized on resin. Anal Chim Acta 426:57
Li J, Tan SN, Ge HL (1996) Silica sol-gel immobilized amperometric biosensor for hydrogen peroxide. Anal Chim Acta 335:137
Garguilo MG, Huynh N, Proctor A, Michael AC (1993) Amperometric sensors for peroxide, choline, and acetylcholine based on electron transfer between horseradish peroxidase and a redox polymer. Anal Chem 65:523
Xiao Y, Ju HX, Chen HY (1999) A reagentless hydrogen peroxide sensor based on incorporation of horseradish peroxidase in poly(thionine) film on a monolayer modified electrode. Anal Chim Acta 391:299
Mao L, Osborne PG, Yamamoto K, Kato T (2002) Continuous on-line measurement of cerebral hydrogen peroxide using enzyme-modified ring–disk plastic carbon film electrode. Anal Chem 74:3684
Updike SJ, Hicks GP (1967) The enzyme electrode. Nature 214:986
Lindgren A, Ruzgas T, Gorton L, Csoregi E, Bautista A, Sakharov G, Gazaryan IY (2000) Biosensors based on novel peroxidases with improved properties in direct and mediated electron transfer. Biosens Bioelectron 15:491
Serradilla SR, Lopez B, Mora D, Mark HB, Kauffmann JM (2002) Hydrogen peroxide sensitive amperometric biosensor based on horseradish peroxidase entrapped in a polypyrrole electrode. Biosens Bioelectron 17:921
Karyakin AA, Karyakina E (1999) Prussian Blue-based ‘artificial peroxidase’ as a transducer for hydrogen peroxide detection. Application to biosensors. Sens Actuators B 57:268
Ricci F, Amine A, Tuta CS, Ciucu AA, Lucarelli F, Palleschi G, Moscone D (2003) Prussian Blue and enzyme bulk-modified screen-printed electrodes for hydrogen peroxide and glucose determination with improved storage and operational stability. Anal Chim Acta 485:111
Ricci F, Palleschi G (2005) Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens Bioelectron 21:389
Buster HJ, Schwarzenbach D, Petter W, Ludi A (1977) The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3.xH2O. Inorg Chem 16:2704
Razmi H, Mohammad-Rezaei R, Heidari H (2009) Self-assembled prussian blue nanoparticles based electrochemical sensor for high sensitive determination of H2O2 in acidic media. Electroanalysis 21:2355
Somani PR, Radhakrishnan S (2003) Electrochromic materials and devices: present and future. Mater Chem Phys 77:117
Zhou PH, Xue DS, Luo HQ, Chen XG (2002) Fabrication, structure, and magnetic properties of highly ordered prussian blue nanowire arrays. Nano Lett 2:845
Nakatani K, Yu P (2001) Photochromic magnetic materials. Adv Mater 13:1411
Beseth PA, Sokol JJ, Shores MP, Heinrich JL, Long JR (2000) High-nuclearity metal-cyanide clusters: assembly of a Cr8Ni6(CN)24 cage with a face-centered cubic geometry. J Am Chem Soc 122:9655
Pan KC, Chuang CS, Cheng SH, Su YO (2001) Electrocatalytic reactions of nitric oxide on Prussian blue film modified electrodes. J Electroanal Chem 501:160
Razmi H, Mohammad-Rezaei R (2010) Flow injection amperometric determination of pyridoxine at a Prussian blue nanoparticle-modified carbon ceramic electrode. Electrochim Acta 50:1814
Pyrasch M, Toutianoush A, Jin WQ, Schnepf J, Tieke B (2003) Self-assembled films of prussian blue and analogues: optical and electrochemical properties and application as ion-sieving membranes. Chem Mater 15:245
Jayalakshimi M, Scholz F (2000) Charge–discharge characteristics of a solid-state Prussian blue secondary cell. J Power Sources 87:212
Moscone D, D’Ottavi D, Compagnone D, Palleschi G, Amine A (2001) Construction and analytical characterization of prussian blue-based carbon paste electrodes and their assembly as oxidase enzyme sensors. Anal Chem 73:2529
Culp JT, Park JH, Stratakis D, Meisel MW, Talham DR (2002) Supramolecular assembly at interfaces: formation of an extended two-dimensional coordinate covalent square grid network at the air-water interface. J Am Chem Soc 124:10083
Neff VD (1978) Electrochemical oxidation and reduction of thin films of Prussian Blue. J Electrochem Soc 125:886
Pournaghi-Azar MH, Dastangoo H (2002) Electrochemical characteristics of an aluminum electrode modified by a palladium hexacyanoferrate film, synthesized by a simple electroless procedure. J Electroanal Chem 523:26
Lezna RO, Romagnoli R, Tacconi NR, Rajeshwar K (2003) Spectroelectrochemistry of palladium hexacyanoferrate films on platinum substrates. J Electroanal Chem 544:101
Czirók E, Bácskai J, Kulesza PJ, Inzelt G, Wolkiewicz A, Miecznikowski K, Malik MA (1996) Quartz crystal microbalance study of the growth of indium hexacyanoferrate films during electrodeposition and coagulation. J Electroanal Chem 405:205
Wang Y, Zhu GY, Wang EK (1997) Electrochemical quartz crystal microbalance study for vanadium hexacyanoferrates: monitoring of film growth and ion effects during redox reactions. J Electroanal Chem 430:127
Kasem K, Steldt FR, Miller TJ, Zimmerman AN (2003) Electrochemical synthesis of zeolite-like ruthenium-based hexacyanometalates multi-film assemblies. Microporous Mesoporous Mater 66:133
Bacskai J, Martinusz K, Czirok E, Inzelt G, Kulesza PJ, Malik MA (1995) Polynuclear nickel hexacyanoferrates: monitoring of film growth and hydrated counter-cation flux/storage during redox reactions. J Electroanal Chem 385:241
Sheng QL, Yu H, Zheng J (2007) Sol–gel derived terbium hexacyanoferrate modified carbon ceramic electrode: Electrochemical behavior and its electrocatalytical oxidation of ascorbic acid. J Electroanal Chem 606:39
Pournaghi-Azar MH, Nahalparvari H (2005) Zinc hexacyanoferrate film as an effective protecting layer in two-step and one-step electropolymerization of pyrrole on zinc substrate. Electrochim Acta 50:2107
Lin MS, Tseng TF, Shih WC (1998) Chromium(III) hexacyanoferrate(II)-based chemical sensor for the cathodic determination of hydrogen peroxide. Analyst 123:159
Gao Z, Zhang Y, Wang G (1998) Electrochemistry of a thin cobalt(II)– heptacyanonitrosylferrate film modified glassy carbon electrode. Anal Sci 14:1053
Pournaghi-Azar MH, Razmi-Nerbin H (2001) Electrocatalytic characteristics of thiosulfate oxidation at nickel plated aluminum electrode modified with nickel pentacyanonitrosylferrate films. Electroanalysis 13:465
Razmi H, Heidari H (2009) Amperometric determination of hydrogen peroxide on surface of a novel PbPCNF-modified carbon-ceramic electrode in acidic medium. J Electroanal Chem 625:101
Razmi H, Es H (2009) Nanomolar detection of hydrogen peroxide at a new polynuclear cluster of tin pentacyanonitrosylferrate nanoparticle-modified carbon ceramic electrode. Anal Biochem 392:126
Gun G, Tsionsky M, Lev O (1994) Voltammetric studies of composite ceramic carbon working electrodes. Anal Chim Acta 294:261
Pamidi PVA, Parrado C, Kane SA, Wang J, Smyth MR, Pingarn JM (1997) Sol– gel carbon composite electrode as an amperometric detector for liquid chromatography. Talanta 44:1929
Tsionsky M, Gun G, Glezer V, Lev O (1994) Sol–gel-derived ceramic-carbon composite electrodes: introduction and scope of applications. Anal Chem 66:1747
Lezna RO, Romagnoli R, Tacconi NR, Rajeshwar K (2002) Cobalt hexacyanoferrate: compound stoichiometry, infrared spectroelectrochemistry, and photoinduced electron transfer. J Phys Chem B 106:3612
Xun Z, Cai C, Lu T (2004) Effects of a surfactant on the electrocatalytic activity of cobalt hexacyanoferrate modified glassy carbon electrode towards the oxidation of dopamine. Electroanalysis 16:674
Pournaghi-Azar MH, Nahalparvari H (2005) Preparation and characterization of electrochemical and electrocatalytic behavior of a zinc pentacyanonitrosylferrate film-modified glassy carbon electrode. J Electroanal Chem 583:307
Carapuça HM, Simao JEJ, Fogg AG (1998) Comproportionation and disproportionation reactions in the electrochemical reduction of nitroprusside at a hanging mercury drop electrode in acidic solution. J Electroanal Chem 455:93
Chen SM (2002) Preparation, characterization, and electrocatalytic oxidation properties of iron, cobalt, nickel, and indium hexacyanoferrate. J Electroanal Chem 521:29
Murray RW (1984) In: Bard AJ (ed) Electroanalytical Chemistry, vol. 13. Marcel Dekker, New York (Chapter 3)
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19
Feldman BJ, Murray RW (1987) Electron diffusion in wet and dry Prussian blue films on interdigitated array electrodes. Inorg Chem 26:1702
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications. Wiley, New York
Galus Z (1976) Fundamentals of Electrochemical Analysis, Chapter 10. Ellis Horwood, New York
Li M, Zhao G, Yue Z, Huang S (2009) Sensor for traces of hydrogen peroxide using an electrode modified by multiwalled carbon nanotubes, a gold-chitosan colloid, and Prussian blue. Microchim Acta 167:167
Wang Q, Yun Y, Zheng J (2009) Nonenzymatic hydrogen peroxide sensor based on a polyaniline-single walled carbon nanotubes composite in a room temperature ionic liquid. Microchim Acta 167:153
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Razmi, H., Mohammad-Rezaei, R. Non-enzymatic hydrogen peroxide sensor using an electrode modified with iron pentacyanonitrosylferrate nanoparticles. Microchim Acta 171, 257–265 (2010). https://doi.org/10.1007/s00604-010-0426-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0426-x