Abstract
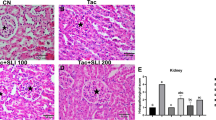
The immunosuppressive drug, cyclosporine A (CsA), has been used in patients who were treated for immune diseases and in transplant patients. However, nephrotoxicity due to CsA, remains an important clinical challenge. Although the mechanisms of nephrotoxicity are far from clear, notwithstanding there is evidence that suggests the role of oxidative stress in its pathogenesis. This study was conducted to investigate the protective effect of the Chinese herbal medicine, astragalus membranaceus (AM), in CsA-induced nephrotoxicity in a rat model. Adult Sprague–Dawley rats were treated with olive oil, CsA (25 mg/kg/bw), AM (5 mg/kg/bw), CsA plus AM for 30 days. Renal function, Malondialdehyde (MDA) levels were measured and histopathology, and Immunohistochemical staining for Endothelin was performed. In this study, CsA caused a significant deterioration in renal function, morphology, and also induced oxidative stress, as indicated by increased renal MDA. Administration of AM along with CsA improved the functional and histological parameters of the kidneys, and counteracted the oxidative stress induced by CsA. These results suggested that AM has a protective potential in experimental CsA nephrotoxicity.


Similar content being viewed by others

References
Mason J. The pathophysiology of sandimmun (cyclosporine) in man and animals. Pediatr Nephrol. 1990;4:554–74.
Khan M, Shobha JC, Mohan IK, Rao Naidu MU, Prayag A, Kutala VK. Spirulina attenuates cyclosporine-induced nephrotoxicity in rats. J Appl Toxicol. 2006;26(5):444–51.
Rezzani R, Rodella L, Bianchi R. Induction of endothelin in rat kidney after cyclosporine A treatment. Acta Histochem. 2001;103:423–31.
Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993;91:2144–9.
Serino F, Grevel J, Napoli KL, Kahan BD, Strobel HW. Oxygen radical formation by the cytochrome P450 system as a cellular mechanism for cyclosporine toxicity. Transplant Proc. 1994;26:2916–7.
Amore A, Gianoglio B, Ghigo D, Peruzzi L, Porcellini MG, Bussolino F, et al. A possible role for nitric oxide in modulating the functional cyclosporine toxicity by arginine. Kidney Int. 1995;47:1507–14.
Dusting GJ, Akita K, Hickey H, Smith M, Gurevich V. Cyclosporine A and tacrolimus (FK506) suppress expression of inducible nitric oxide synthase in vitro by different mechanisms. Br J Pharmacol. 1999;128:337–44.
Buetler TM, Cottet-Maire F, Krauskopf A, Ruegg UT. Does cyclosporine A generate free radicals? Trends Pharmacol Sci. 2000;21:288.
Burdmann EA, Andoh TF, Yu L, Bennett WM. Cyclosporine nephrotoxicity. Semin Nephrol. 2003;23(5):365–76.
Cho WC, Leung KN. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol. 2007;113:132–41.
Su L, Chen YC, Hu JD, et al. Comparisons between different doses of Astragalus membranaceus and Salvia miltiorrhiza in rats proteinuria. Chinese Journal of New Drugs and Clinical Remedies. 2000;19:205–8.
Shi JF, Zhu HW, Zhang C, et al. Therapeutic effect of astragalus on patients with chronic glomerulonephritis. Acta Medicinalis Secondae Shanghai. 2002;22:245–8.
Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2(3):247–67.
Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst Rev. 2005;25(1):CD004540.
Farthing MJ, Clark ML. Nature of the toxicity of cyclosporine A in the rat. Biochem Pharmacol. 1981;30(24):3311–6.
Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuate cyclosporine-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14(4):923–9.
Amudha G, Josephine A, Varalakshmi P. Role of lipoic acid in reducing the oxidative stress induced by cyclosporine A. Clin Chim Acta. 2006;372(1–2):134–9.
Hagar HH, El Etter E, Arafa M. Taurine attenuates hypertension and renal dysfunction induced by cyclosporine A in rats. Clin Exp Pharmacol Physiol. 2006;33(3):189–96.
Caline RY, White DJG, Thiru S, Evans DB, MCMaster P, Dunn DC, et al. Cyclosporine A in patients reciving renal allografts from cadaver donors. Lancet. 1978;II:1323–7.
Wongmekiat O, Leelarugrayub N, Thamprasert K. Beneficial effect of shallot (Allium ascalonicum L.) extract on cyclosporine nephrotoxicity in rats. Food Chem Toxicol. 2008;46:1844–50.
Parra CT, Conejo Garcia JR, Carballo AF, de Arriba G. Antioxidant nutrients protect against cyclosporine A nephrotoxicity. Toxicology. 2003;189:99–111.
Zhong Z, Connor HD, Yin M, Moss N, Mason RP, Bunzendahl H, et al. Dietary glycine and renal denervation prevents cyclosporin A-induced hydroxyl radical production in rat kidney. Mol Pharmacol. 1999;56(3):455–63.
Yoshimura R, Yoshimura N, Nakatani T, Kusunose E, Yamaguchi T, Oka T, et al. The effect of cyclosporine on renal microsomal cytochrome P-450 system. Clin Nephrol. 1993;40:339–45.
Freeman BA, Crapho JD. Biology of disease: free radical and tissue injury. Lab Invest. 1982;47:412–26.
Anjaneyulu M, Tirkey N, Chopra K. Attenuation of cyclosporine-induced renal dysfunction by catechin: possible antioxidant mechanism. Ren Fail. 2003;25:691–707.
Padi SS, Chopra K. Salvage of cyclosporine A-induced oxidative stress and renal dysfunction by carvedilol. Nephron. 2002;92(3):685–92.
Nakahama H. Stimulatory effect of cyclosporine A on endothelin secretion by a cultured renal epithelial cell line, LLC-PK cells. Eur J Pharmacol. 1990;180:191–2.
Textor SC, Burnett Jr JC, Romero JC, et al. Urinary endothelin and renal vasoconstriction with cyclosporine or FK506 after liver transplantation. Kidney Int. 1995;47:1426–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Kenawy, A.EM. Investigating the Protective Effects of Astragalus Membranaceus on Nephrotoxicity in Cyclosporine A-treated Rats. Kidney 19, 119–125 (2010). https://doi.org/10.1007/s00596-009-0136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00596-009-0136-8