Abstract
Purpose
This analysis was performed to clarify the usefulness of skeletal muscle measurements using computed tomography (CT) in patients with esophageal cancer and the effect of treatment-induced changes in the skeletal muscle mass on the prognosis.
Methods
Ninety-seven male patients who underwent thoracoscopic esophagectomy for esophageal squamous cell carcinoma were included in the study. The preoperative CT images were analyzed retrospectively.
Results
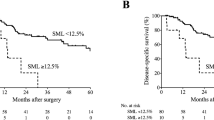
In a survival analysis performed according to the preoperative data of skeletal muscle, the low-skeletal muscle index (l-SMI) group had a poorer outcome than the normal skeletal muscle index (n-SMI) group in terms of both the overall survival (OS) and the relapse-free survival (RFS) (OS: P < 0.01, RFS: P = 0.01). In the multivariate analysis for the OS, preoperative l-SMI was an independent predictor (hazard ratio: 3.68, 95% confidence interval 1.32–10.2, P = 0.01). In patients who underwent neoadjuvant therapy (NAT), the SMI was significantly reduced after NAT (P < 0.01). The preoperative skeletal muscle area on CT was strongly correlated with the results of a bioelectrical impedance analysis (BIA) (ρ = 0.77, P < 0.01).
Conclusions
A decreased preoperative skeletal muscle mass was associated with a poor outcome. In patients who underwent NAT, the SMI was significantly reduced after NAT. An analysis of the skeletal muscle mass using CT images was found to be useful for providing data that corresponded with BIA data.






Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Watanabe M, Tachimori Y, Oyama T, Toh Y, Matsubara H, Ueno M, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1–24.
Klassen P, Baracos V, Gramlich L, Nelson G, Mazurak V, Martin L. Computed-tomography body composition analysis complements pre-operative nutrition screening in colorectal cancer patients on an enhanced recovery after surgery pathway. Nutrients. 2020;12:3745.
Kagifuku Y, Tohara H, Wakasugi Y, Susa C, Nakane A, Toyoshima M, et al. What factors affect changes in body composition and swallowing function in patients hospitalized for oral cancer surgery? Clin Interv Aging. 2020;15:1–7.
Velho S, Santos MPC, Cunha C, Agostinho L, Cruz R, Costa F, et al. Body composition influences post-operative complications and 90-day and overall survival in pancreatic surgery patients. GE-Portuguese J Gastroenterol. 2021;28:11–23.
Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K. Prognostic significance of sarcopenia in patients undergoing esophagectomy for superficial esophageal squamous cell carcinoma. Dis Esophagus. 2019;32:1–9.
Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29:627–33.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63.
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–9.
Chien M, Huang T, Wu Y. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56:1710–5.
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21.
Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
Abbass T, Ho Y, Horgan P, Dolan R, McMillan D. The relationship between computed tomography derived skeletal muscle index, psoas muscle index and clinical outcomes in patients with operable colorectal cancer. Clin Nutr ESPEN. 2020;39:104–13.
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder K, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–8.
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3:e172319–e172319.
Durham WJ, Dillon EL, Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2009;12:72–7.
Ida S, Watanabe M, Karashima R, Imamura Y, Ishimoto T, Baba Y, et al. Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol. 2014;21:3675–9.
Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barber A, et al. β-Hydroxy-β-methylbutyrate supplementation in critically ill trauma patients. J Trauma Acute Care Surg. 2007;62:125–32.
May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–9.
Berk L, James J, Schwartz A, Mahadevan A, Samuels M, Kachnic L, et al. A randomized, double-blind, placebo-controlled trial of a β-hydroxyl β-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer. 2008;16:1179–88.
Acknowledgements
The analysis of the CT findings in this study was done with the support of the Department of Radiological Technology, Tokai University Hospital. We thank Mr. Yuuki Sato, Mr. Yasushi Katsunuma, and Mr. Katsuhiro Watanabe. We also thank Ms. Izu Inada for her support with the data analysis.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamamoto, M., Ozawa, S., Koyanagi, K. et al. Usefulness of skeletal muscle measurement by computed tomography in patients with esophageal cancer: changes in skeletal muscle mass due to neoadjuvant therapy and the effect on the prognosis. Surg Today 53, 692–701 (2023). https://doi.org/10.1007/s00595-023-02657-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02657-1