Abstract
Purpose
The controlling nutritional status (CONUT) score can be easily calculated from the serum albumin concentration, total cholesterol concentration, and total lymphocyte count. The study aim was to assess the preoperative prognostic factors for the overall survival (OS) of distal cholangiocarcinoma (DCC) following pancreatoduodenectomy (PD) and to demonstrate the utility of the CONUT score.
Methods
A total of 149 consecutive patients who underwent PD for DCC between September 2002 and December 2016 were divided into a low-CONUT (LC) group (CONUT scores ≤ 2) and a high-CONUT (HC) group (CONUT scores ≥ 3). The clinicopathological characteristics and OS of the patients were evaluated retrospectively. Prognostic factors of DCC were identified by multivariate analyses.
Results
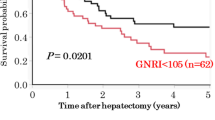
The LC and HC groups included 113 and 36 patients, respectively. The OS was better in the LC group than in the HC group (median survival time and 5 year survival rate: 82 months and 56.8% vs. 38 months and 27.6%, P = 0.005). Multivariate analyses for the OS in all patients showed that the tumor differentiation, perineural invasion, residual tumor status, portal vein resection, blood transfusion, and preoperative CONUT score ≥ 3 were independently associated with a poor survival.
Conclusion
The CONUT score may be a useful preoperative factor for predicting the long-term survival in patients with DCC.



Similar content being viewed by others
References
Bird NTE, McKenna A, Dodd J, Poston G, Jones R, Malik H. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 2018;105:1408–16.
van der Gaag NA, Kloek JJ, de Bakker JK, Musters B, Geskus RB, Busch OR, et al. Survival analysis and prognostic nomogram for patients undergoing resection of extrahepatic cholangiocarcinoma. Ann Oncol. 2012;23:2642–9.
Courtin-Tanguy L, Rayar M, Bergeat D, Merdrignac A, Harnoy Y, Boudjema K, et al. The true prognosis of resected distal cholangiocarcinoma. J Surg Oncol. 2016;113(575):580.
Andrianello S, Paiella S, Allegrini V, Ramera M, Pulvirenti A, Malleo G, et al. Pancreaticoduodenectomy for distal cholangiocarcinoma: surgical results, prognostic factors, and long-term follow-up. Langenbecks Arch Surg. 2015;400:623–8.
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45.
Cabre M, Ferreiro C, Arus M, Roca M, Palomera E, Serra-Prat M. Evaluation of CONUT for clinical malnutrition detection and short-term prognostic assessment in hospitalized elderly people. J Nutr Health Aging. 2015;19:729–33.
Toyokawa G, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. 2017;9:2942–51.
Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC cancer. 2016;16:722.
Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204–12.
Harimoto N, Yoshizumi T, Inokuchi S, Itoh S, Adachi E, Ikeda Y, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. 2018;25:3316–23.
Miyata T, Yamashita YI, Higashi T, Taki K, Izumi D, Kosumi K, et al. The prognostic impact of controlling nutritional status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg. 2018;42:1085–91.
Kato Y, Yamada S, Suenaga M, Takami H, Niwa Y, Hayashi M, et al. Impact of the controlling nutritional status score on the prognosis after curative resection of pancreatic ductal adenocarcinoma. Pancreas. 2018;47:823–9.
Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. The preoperative controlling nutritional status (CONUT) score is an independent prognostic marker for pancreatic ductal adenocarcinoma. Updates Surg. 2020. https://doi.org/10.1007/s13304-020-00792-9.
Kumamoto Y, Kaizu T, Tajima H, Nishizawa N, Ei S, Igarashi K, et al. Neutrophil-to-lymphocyte ratio as a predictor of postoperative morbidity in patients with distal cholangiocarcinoma. Molecular and clinical oncology. 2018;9:362–8.
Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, et al. Modified blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014;18:1108–15.
Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J surg. 2018;105:192–202.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584–91.
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study group of pancreatic surgery (ISGPS). Surgery. 2007;142:761–8.
Lin Y. Robust inference for responder analysis: innovative clinical trial design using a minimum p-value approach. Contemp Clin Trials Commun. 2016;3:65–9.
Vanniyasingam T, Rodseth RN, Lurati Buse GA, Bolliger D, Burkhart CS, Cuthbertson BH, et al. Predicting the occurrence of major adverse cardiac events within 30 days of a vascular surgery: an empirical comparison of the minimum p value method and ROC curve approach using individual patient data meta-analysis. Springerplus. 2016;5:304.
McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41:64–9.
Wu N, Chen G, Hu H, Pang L, Chen Z. Low pretherapeutic serum albumin as a risk factor for poor outcome in esophageal squamous cell carcinomas. Nutr Cancer. 2015;67:481–5.
Cengiz O, Kocer B, Surmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma? Med Sci Monit. 2006;12(6):CR240–CR247.
Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381–9.
Peters SJ, Vanhaecke T, Papeleu P, Rogiers V, Haagsman HP, van Norren K. Co-culture of primary rat hepatocytes with rat liver epithelial cells enhances interleukin-6-induced acute-phase protein response. Cell Tissue Res. 2010;340:451–7.
Honda H, Qureshi AR, Heimburger O, Barany P, Wang K, Pecoits-Filho R, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–48.
Shen J, Wen T, Li C, Yan L, Li B, Yang J. The prognostic prediction role of preoperative serum albumin level in patients with intahepatic cholangiocarcinoma following hepatectomy. Dig Dis. 2018;36:306–13.
Waghray A, Sobotka A, Marrero CR, Estfan B, Aucejo F, Narayanan Menon KV. Serum albumin predicts survival in patients with hilar cholangiocarcinoma. Gastroenterology report. 2017;5:62–6.
Okuyama H, Ichikawa Y, Sun Y, Hamazaki T, Lands WE. Cancer and all-cause mortalities are lower in the higher total cholesterol groups among general populations. World Rev Nutr Diet. 2007;96:37–54.
Roch AM, House MG, Cioffi J, Ceppa EP, Zyromski NJ, Nakeeb A, et al. Significance of portal vein invasion and extent of invasion in patients undergoing pancreatoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2016;20(3):479–87.
Li JR, Zhang Y, Zheng JL. Decreased pretreatment serum cholesterol level is related with poor prognosis in resectable non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8:11877–83.
Wang J, Wang WJ, Zhai L, Zhang DF. Association of cholesterol with risk of pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:3711–9.
Kang R, Li P, Wang T, Li X, Wei Z, Zhang Z, et al. Apolipoprotein E epsilon 2 allele and low serum cholesterol as risk factors for gastric cancer in a Chinese Han population. Sci Rep. 2016;6:19930.
Niendorf A, Nagele H, Gerding D, Meyer-Pannwitt U, Gebhardt A. Increased LDL receptor mRNA expression in colon cancer is correlated with a rise in plasma cholesterol levels after curative surgery. Int J Cancer. 1995;61:461–4.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–7.
Liang L, Zhu J, Jia H, Huang L, Li D, Li Q, et al. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget. 2016;7:1014–28.
Yamada M, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. Microscopic venous invasion in pancreatic cancer. Ann Surg Oncol. 2018;25:1043–51.
Hu LS, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, et al. Impact of microvascular invasion on clinical outcomes after curative-intent resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2019;119:21–9.
Martin D, Joliat GR, Halkic N, Demartines N, Schafer M. Perioperative nutritional management of patients undergoing pancreatoduodenectomy: an international survey among surgeons. HPB (Oxford). 2019;22(1):75–82.
Adiamah A, Skorepa P, Weimann A, Lobo DN. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Ann Surg. 2019;270:247–56.
Ebata T, Ercolani G, Alvaro D, Ribero D, Di Tommaso L, Valle JW. Current status on cholangiocarcinoma and gallbladder cancer. Liver Cancer. 2016;6:59–655.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Fumihiro Terasaki and the other co-authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Terasaki, F., Sugiura, T., Okamura, Y. et al. Use of preoperative controlling nutritional status (CONUT) score as a better prognostic marker for distal cholangiocarcinoma after pancreatoduodenectomy. Surg Today 51, 358–365 (2021). https://doi.org/10.1007/s00595-020-02098-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02098-0