Abstract
Purpose
A phase clinical II trial was conducted to determine the antitumor activity and toxicity of weekly paclitaxel administered to patients with advanced gastric cancer.
Methods
Sixty-eight patients with advanced gastric cancer and performance status 0–2 were treated with 80 mg/m2 paclitaxel over 1 h following a short course of premedication with dexamethasone, diphenhydramine, and ranitidine administered 30 min prior to the delivery of the paclitaxel. In principle, the treatment was repeated weekly for three courses, followed by a 1-week rest.
Results
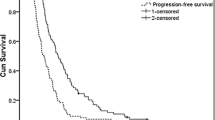
Objective responses were observed in 12 of 68 patients (17.6%; 95% confidence interval: 9.5%–28.8%). Two of fourteen (14.2%) patients with no prior chemotherapy and 10 of 54 (18.5%) patients previously treated for metastatic disease developed a partial response. The median therapeutic duration and the median survival time were 96 days and 222 days, respectively. In 212 (85.5%) of 248 total cycles, the original dose of 80 mg/m2 of paclitaxel was administered and was well tolerated. Fourteen of 68 patients (20.1%) experienced grade 3 or 4 neutropenia. Grade 1 or 2 peripheral neuropathy occurred in 10 patients (14.7%).
Conclusion
Weekly paclitaxel therapy is an active and safe treatment for advanced gastric cancer.
Similar content being viewed by others
References
Cancer Statistics in Japan 2007. In: The Editorial Board of the Cancer Statistics in Japan, editors. Foundation for Promotion of Cancer Research (FPCR); 2007. p. 12–38.
Cutsem EV, Haller D, Ohtsu A. The role of chemotherapy in the current treatment of gastric cancer. Gastric Cancer 2002;5(suppl 1):17–22.
Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomized comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587–591.
Murad AM, Santiago FF, Petroianu A, Rocha PRS, Rodrigues MAG, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72:37–41.
Glimelius B, Hoffman K, Haglund U, Nyrén O, Sjödén PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol 1994;5:189–190.
Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Japan Clinical Oncology Group Study (JCOG9205). Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer. J Clin Oncol 2003;21:54–59.
Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 1999;80:269–272.
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 2000;18:2648–2657.
Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002;20:1996–2004.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–4997.
Boku N, Yamamoto S, Shirao K, Doi T, Sawaki A, Koizumi W, et al. Gastrointestinal Oncology Study Group/Japan Clinical Oncology Group. Randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG9912). J Clin Oncol 2007;25(suppl):LBA4513.
Narahara H, Koizumi W, Hara T, Takagane A, Akiya T, Takagi M, et al. TS-1 Advanced Gastric Cancer (AGC) Clinical Trial Group. Randomized phase III study of S-1 alone versus S-1 + cisplatin in the treatment for advanced gastric cancer (The SPIRITS trial) SPIRITS: S-1 plus cisplatin vs S-1 in RCT in the treatment for stomach cancer. J Clin Oncol 2007;25(suppl):4514.
Sugimachi K, Maehara Y. A phase II trial of a new 5-fluorouracil derivative, BOF-A2 (Emitefur), for patients with advanced gastric cancer. Surg Today 2000;30:1067–1072.
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971;93:2325–2327.
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979;277:665–667.
Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst 1990;82:1247–1259.
Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 1991;83:1797–1805.
Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J Clin Oncol 1992;10:1748–1753.
Einzig AI, Hochster H, Wiernik PH, Trump DL, Dutcher JP, Garowski E, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs 1991;9:59–64.
Chang AY, Kim K, Glick J, Anderson T, Karp D, Johnson D. Phase II study of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer: The Eastern Cooperative Oncology Group Results. J Natl Cancer Inst 1993;85:388–394.
Yamada Y, Shirao K, Ohtsu A, Boku N, Hyodo I, Saitoh H, et al. Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol 2001;12:1133–1137.
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME, et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol 1994;12:2654–2666.
Kodera Y, Ito S, Mochizuki Y, Fujitake S, Koshikawa K, Kanyama Y, et al. A phase II study of weekly paclitaxel as second-line chemotherapy for advanced gastric Cancer (CCOG0302 study). Anticancer Res 2007;27:2667–2671.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–216.
Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. Part IV. Response assessment of chemotherapy for gastric carcinoma. 1st English ed. Tokyo: Kanehara; 1995. p. 90–104.
Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF. Phase II study of Taxol in patients with advanced gastric carcinoma. Cancer J Sci Am 1998;4:269–274.
Cascinu S, Graziano F, Cardarelli N, Marcellini M, Giordani P, Menichetti ET, et al. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs 1998;9:307–310.
Garcia AA, Leichman CG, Lenz HJ, Baranda J, Lujan R, Casagrande Y, et al. Phase II trial of outpatient schedule of paclitaxel in patients with previously untreated metastatic, measurable adenocarcinoma of the stomach. Jpn J Clin Oncol 2001;31:275–278.
Yamaguchi K, Tada M, Horikoshi N, Otani T, Takiuchi H, Saitoh S, et al. Phase II study of paclitaxel with 3-h infusion in patients with advanced gastric cancer. Gastric Cancer 2002;5:90–95.
Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, et al. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 1998;16:3353–3361.
Kimura M, Koida T, Yanagita Y. Weekly administration of paclitaxel for advanced or metastatic breast cancer-short-course premedication for outpatients (in Japanese with English abstract). Gan To Kagaku Ryoho (Jpn J Cancer Chemother) 2000;27:1703–1708.
Fidias P, Supko JG, Martins R, Boral A, Carey R, Grossbard M, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res 2001;12:3942–3949.
Markman M, Hall J, Spitz D, Weiner S, Carson L, Van Le L, et al. Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refractory ovarian cancer. J Clin Oncol 2002;20(9):2365–2369.
Kim R, Osaki A, Toge T. Pharmacokinetic and biochemical analysis in the treatment of weekly paclitaxel in relapsed breast cancer. Oncol Rep 2001;8:1171–1176.
Rosenberg P, Andersson H, Boman K, Ridderheim M, Sorbe B, Puistola U, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with per oral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol 2002;41:418–424.
Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 2005;23:5983–5992.
Tahara M, Ohtsu A, Boku N, Nagashima F, Muto M, Sano Y, et al. Sequential methotrexate and 5-fluorouracil therapy for gastric cancer patients with peritoneal dissemination: a retrospective study. Gastric Cancer 2001;4:212–218.
Sakamoto J, Morita S, Yumiba T, Narahara H, Kinoshita K, Nakane Y, et al. A phase II clinical trial to evaluate the effect of paclitaxel in patients with ascites caused by advanced or recurrent gastric carcinoma: a new concept of clinical benefit response for non-measurable type of gastric cancer. Jpn J Clin Oncol 2003;33:238–240.
Hagiwara A, Takahashi T, Kojima O, Sawai K, Yamaguchi T, Yamane T, et al. Prophylaxis with carbon-adsorbed mitomycin against peritoneal recurrence of gastric cancer. Lancet 1992;339(8794):629–631.
Adachi W, Koike S, Rafique M, Kajikawa S, Kaneko G, Kuroda T, et al. Preoerative intraperitoneal chemotherapy for gastric cancer, with special reference to delayed peritoneal complications. Surg Today 1995;25:396–403.
O’Boyle KP, Wang Y, Schwartz EL, Regl DL, Einzig A, Dutcher JP, et al. Development of two radioimmunoassays to detect paclitaxel in sera and in cerebrospinal, ascitic, and pleural fluids. Cancer 1997;79:1022–1030.
Kobayashi M, Sakamoto J, Namikawa T, Okamoto K, Okabayashi T, Ichikawa K, et al. Pharmacokinetic study of paclitaxel in malignant ascites from advanced gastric cancer patients. World J Gastroenterol 2006;12:1412–1415.
Ishida T, Shimokawa H, Kawaguchi K, Nose N, Ikegami T, Itoh H, et al. Effective weekly paclitaxel administration for gastric cancer with malignant ascites — a case report (in Japanese with English abstract). Gan To Kagaku Ryoho (Jpn J Cancer Chemother) 2002;29:1643–1646.
Kobayashi M, Oba K, Sakamoto J, Kondo K, Nagata N, Okabayashi T, et al. Pharmacokinetic study of weekly administration dose of paclitaxel in patients with advanced or recurrent gastric cancer in Japan. Gastric Cancer 2007;10:52–57.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Emi, Y., Yamamoto, M., Takahashi, I. et al. Phase II study of weekly paclitaxel by one-hour infusion for advanced gastric cancer. Surg Today 38, 1013–1020 (2008). https://doi.org/10.1007/s00595-008-3769-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-008-3769-8