Abstract
Aims
Disturbance of intestinal homeostasis promotes the development of type 2 diabetes. Although intensive insulin therapy has been shown to promote extended glycemic remission in newly diagnosed type 2 diabetic patients through multiple mechanisms, its effect on intestinal homeostasis remains unknown.
Methods
This study evaluated the effects of intensive insulin therapy on intestinal morphometric parameters in a hyperglycemic mice model induced by high-fat diet (HFD). 16S rRNA V4 region sequencing and multivariate analysis were utilized to evaluate the structural changes of gut microbiota.
Results
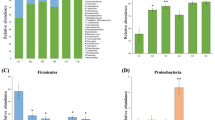
HFD-induced increases in the lengths of villus, microvillus and crypt depth were significantly reversed after intensive insulin therapy. Moreover, intestinal proliferation was notably decreased after intensive insulin therapy, whereas intestinal apoptosis was further increased. Importantly, intensive insulin therapy significantly shifted the overall structure of the HFD-disrupted gut microbiota toward that of mice fed a normal diet and changed the gut microbial composition. The abundances of 54 operational taxonomic units (OTUs) were changed by intensive insulin therapy. Thirty altered OTUs correlated with two or more intestinal morphometric parameters and were designated ‘functionally relevant phylotypes.’
Conclusions
For the first time, our data indicate that intensive insulin therapy recovers diabetes-associated gut structural abnormalities and restores the microbiome landscape. Moreover, specific altered ‘functionally relevant phylotypes’ correlates with improvement in diabetes-associated gut structural alterations.






Similar content being viewed by others
References
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98. https://doi.org/10.1038/nrendo.2017.151
Weng J, Li Y, Xu W et al (2008) Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371(9626):1753–1760. https://doi.org/10.1016/S0140-6736(08)60762-X
Hu Y, Li L, Xu Y et al (2011) Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and beta-cell function in subjects with long-term remission. Diabetes Care 34(8):1848–1853. https://doi.org/10.2337/dc10-2105
Nathan DM, Buse JB, Davidson MB et al (2006) Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 29(8):1963–1972. https://doi.org/10.2337/dc06-9912
Bravi MC, Armiento A, Laurenti O et al (2006) Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metab Clin Exp 55(5):691–695. https://doi.org/10.1016/j.metabol.2006.01.003
Kaneto H, Nakatani Y, Kawamori D et al (2005) Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int J Biochem Cell Biol 37(8):1595–1608. https://doi.org/10.1016/j.biocel.2005.04.003
Juurinen L, Tiikkainen M, Hakkinen AM, Hakkarainen A, Yki-Jarvinen H (2007) Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 292(3):E829–E835. https://doi.org/10.1152/ajpendo.00133.2006
LeRoith D, Fonseca V, Vinik A (2005) Metabolic memory in diabetes–focus on insulin. Diabetes Metab Res Rev 21(2):85–90. https://doi.org/10.1002/dmrr.530
Bi Y, Sun WP, Chen X et al (2008) Effect of early insulin therapy on nuclear factor kappaB and cytokine gene expressions in the liver and skeletal muscle of high-fat diet, streptozotocin-treated diabetic rats. Acta Diabetol 45(3):167–178. https://doi.org/10.1007/s00592-008-0038-7
Petit V, Arnould L, Martin P et al (2007) Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 48(2):278–287. https://doi.org/10.1194/jlr.M600283-JLR200
Scoaris CR, Rizo GV, Roldi LP et al (2010) Effects of cafeteria diet on the jejunum in sedentary and physically trained rats. Nutrition 26(3):312–320. https://doi.org/10.1016/j.nut.2009.04.012
Adachi T, Mori C, Sakurai K, Shihara N, Tsuda K, Yasuda K (2003) Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. Endocr J 50(3):271–279
Troy S, Soty M, Ribeiro L et al (2008) Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8(3):201–211. https://doi.org/10.1016/j.cmet.2008.08.008
Mao J, Hu X, Xiao Y et al (2013) Overnutrition stimulates intestinal epithelium proliferation through beta-catenin signaling in obese mice. Diabetes 62(11):3736–3746. https://doi.org/10.2337/db13-0035
Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP (2002) Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 282(2):G241–G248. https://doi.org/10.1152/ajpgi.00310.2001
Mithieux G, Bady I, Gautier A, Croset M, Rajas F, Zitoun C (2004) Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 286(3):E370–E375. https://doi.org/10.1152/ajpendo.00299.2003
Verdam FJ, Greve JW, Roosta S et al (2011) Small intestinal alterations in severely obese hyperglycemic subjects. J Clin Endocrinol Metab 96(2):E379–E383. https://doi.org/10.1210/jc.2010-1333
Peck BC, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P (2017) Functional transcriptomics in diverse intestinal epithelial cell types reveals robust microRNA sensitivity in intestinal stem cells to microbial status. J Biol Chem 292(7):2586–2600. https://doi.org/10.1074/jbc.M116.770099
Velasquez-Manoff M (2015) Gut microbiome: the peacekeepers. Nature 518(7540):S3–S11. https://doi.org/10.1038/518S3a
Chevalier C, Stojanovic O, Colin DJ et al (2015) Gut microbiota orchestrates energy homeostasis during cold. Cell 163(6):1360–1374. https://doi.org/10.1016/j.cell.2015.11.004
Dossa AY, Escobar O, Golden J, Frey MR, Ford HR, Gayer CP (2016) Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am J Physiol Gastrointest Liver Physiol 310(2):G81–G92. https://doi.org/10.1152/ajpgi.00065.2015
Krishna-Subramanian S, Hanski ML, Loddenkemper C et al (2012) UDCA slows down intestinal cell proliferation by inducing high and sustained ERK phosphorylation. Int J Cancer 130(12):2771–2782. https://doi.org/10.1002/ijc.26336
Zeng H, Claycombe KJ, Reindl KM (2015) Butyrate and deoxycholic acid play common and distinct roles in HCT116 human colon cell proliferation. J Nutr Biochem 26(10):1022–1028. https://doi.org/10.1016/j.jnutbio.2015.04.007
Wang H, Wang X, Zhu Y, Chen F, Sun Y, Han X (2015) Increased androgen levels in rats impair glucose-stimulated insulin secretion through disruption of pancreatic beta cell mitochondrial function. J Steroid Biochem Mol Biol 154:254–266. https://doi.org/10.1016/j.jsbmb.2015.09.003
Feng W, Wang H, Zhang P et al (1861) Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochem Biophys Acta 7:1801–1812. https://doi.org/10.1016/j.bbagen.2017.03.017
Stickle D, Turk J (1997) A kinetic mass balance model for 1,5-anhydroglucitol: applications to monitoring of glycemic control. Am J Physiol 273(4 Pt 1):E821–E830
Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK (2014) Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 155(9):3302–3314. https://doi.org/10.1210/en.2014-1112
Creamer B, Shorter RG, Bamforth J (1961) The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut 2:110–118
Andres SF, Santoro MA, Mah AT et al (2015) Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs. Am J Physiol Gastrointest Liver Physiol 308(2):G100–G111. https://doi.org/10.1152/ajpgi.00287.2014
Salzman NH, Bevins CL (2013) Dysbiosis—a consequence of Paneth cell dysfunction. Semin Immunol 25(5):334–341. https://doi.org/10.1016/j.smim.2013.09.006
Ussar S, Haering MF, Fujisaka S et al (2017) Regulation of glucose uptake and enteroendocrine function by the intestinal epithelial insulin receptor. Diabetes 66(4):886–896. https://doi.org/10.2337/db15-1349
Berr F, Kullak-Ublick GA, Paumgartner G, Munzing W, Hylemon PB (1996) 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology 111(6):1611–1620
Acknowledgements
This work was supported by the National Natural Science Foundation of China Grant Awards (81770819, 81570737, 81370947, 81570736, 81500612, 81400832, 81600637, 81600632 and 81703294), the National Key Research and Development Program of China (2016YFC1304804 and 2017YFC1309605), the Jiangsu Provincial Key Medical Discipline (ZDXKB2016012), the Key Project of Nanjing Clinical Medical Science, Jiangsu Province Key Research and Development Program (BE2016606), the Jiangsu Provincial Medical Talent (ZDRCA2016062), the Natural Science Foundation of Jiangsu Province of China (BK20170125), the Jiangsu Provincial Medical Youth Talent (QNRC2016020, QNRC2016019 and QNRC2016018), the Medical Scientific Research Foundation of Jiangsu Province of China (Z201610 and Q2017006), the Science and Technology Project of Administration of Traditional Chinese Medicine of Jiangsu Province of China (YB2015072), the Six Talent Peaks Project of Jiangsu Province of China (WSN-165 and SWYY-091), the Fundamental Research Funds for the Central Universities (021414380444, 021414380092, 021414380208, 021414380160, 021414380142, 021414380279, 021414380296 and 021414380317), the Nanjing Science and Technology Development Project (ZKX16036, YKK16105 and 201605019) and the Nanjing Health Youth Talent (QRX17123).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal studies were conducted according to guidelines established by the Research Animal Care Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School.
Informed consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection "Gut Microbiome and Metabolic Disorders" managed by Massimo Federici
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Tang, W., Zhang, P. et al. Modulation of gut microbiota contributes to effects of intensive insulin therapy on intestinal morphological alteration in high-fat-diet-treated mice. Acta Diabetol 57, 455–467 (2020). https://doi.org/10.1007/s00592-019-01436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01436-0