Abstract
Summary
This paper presents a comparison of quantitative computed tomography (QCT) and dual-energy X-ray absorptiometry (DXA) in osteoporosis with vertebral fracture and osteoporosis without fracture. It has been proved that the volumetric bone mineral density (vBMD) measured by QCT exhibits a stronger correlation with fracture risk than areal bone mineral density (aBMD) measured by DXA.
Purpose
This study aims to systematically evaluate the ability of QCT and DXA to distinguish between osteoporosis with vertebral fracture and osteoporosis without fracture according to vBMD and aBMD.
Methods
We conducted a primary literature search of the online databases up to 3 July, 2022, in both English and Chinese publications, combining synonyms for “QCT”, “DXA” and “osteoporosis”. The Newcastle–Ottawa scale (NOS) was employed to evaluate the quality of the selected articles. vBMD obtained through QCT and aBMD obtained through DXA were extracted, and were analyzed by Review Manager 5.4 and RStudio.
Results
Six studies with 610 individuals aged 45 to 90, of which 179 had vertebral fractures, were included in the final analysis. The weighted mean difference (WMD) between osteoporosis with vertebral fracture and osteoporosis without fracture for vBMD was − 27.08 (95% CI − 31.24 to − 22.92), while for aBMD was − 0.05 (95% CI − 0.08 to − 0.03).
Conclusions
Both vBMD detected by QCT and aBMD detected by DXA could discriminate fracture status in the spine, and vBMD performed a stronger correlation with fracture risk.
Trial registration: PROSPERO 2022 CRD42022349185.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mass and deterioration of the microstructure of bone tissue, which can lead to an increase in bone fragility and fracture risk, with serious physical, psychosocial, and economic consequences [1, 2]. Osteoporosis can occur in all age groups, with a higher prevalence observed in postmenopausal women and older men [3]. Postmenopausal osteoporosis (Type I) and age-related osteoporosis (Type II) are the most common forms of bone loss in clinical practice [4]. In the early stages of osteoporosis, trabecular bone experiences the primary loss, while cortical bone loss becomes more prominent with advancing age. This transition of bone loss site corresponds to the epidemiology of osteoporotic fracture. The loss of trabecular bone is more common in vertebral compression fractures in individuals under 65 years of age, whereas the loss of cortical bone is associated with hip fractures in those aged 65 and above [5]. During the initial phase of bone loss, bone resorption in the trabecular compartment leads to the thinning or loss of trabeculae, deterioration of the trabecular connections, and widening of trabecular spaces [6]. Decreased bone strength and decaying bone structure contribute to vertebral compression or other fragility fractures [7].
The prevention and treatment of osteoporosis have emerged as significant global public health challenges [8]. As the pathogenesis of osteoporosis is not completely clear and bone loss occurs insidiously, patients who initially show no symptoms mistakenly assume that they do not have osteoporosis, so the early diagnosis of osteoporosis is important [9, 10]. With the advance in technology, dual energy X-ray absorptiometry (DXA) has been applied to the measurement of areal bone mineral density (aBMD), and studies have revealed a progressive increase in fracture risk as BMD diminishes [11, 12]. According to the diagnostic criteria established by the World Health Organization, osteoporosis is defined by BMD measured by DXA [13]. However, the limitations of DXA in diagnosing osteoporosis and assessing fracture risk have been recognized as the understanding of osteoporosis updating. Firstly, spinal segments with severe end-plate sclerosis, osteophytes, and previous compression fractures must be excluded when using T-scores [14]. In addition, DXA cannot provide detailed information about bone microstructure, potentially resulting in the underdiagnosis of osteoporosis [15]. Notably, several studies have indicated that only about half of fracture patients meet the criteria for osteoporosis based on low aBMD [16].
With the increasing number of studies on the application of Quantitative computed tomography (QCT) in osteoporosis diagnosis, QCT has been identified as a supplement or even an alternative to DXA [17]. Quantitative computed tomography (QCT) is a three-dimensional technique for measuring BMD in the axial spine and peripheral bone (forearm, tibia), which not only provides measurements in volumetric bone mineral density (vBMD) units of mg/cm3, but also allows for the selective assessment of trabecular or cortical bone mineral density (BMD) according to the region of interest [18]. Previous studies have demonstrated that QCT is more sensitive than DXA in the detection of bone mineral loss [19].
The purpose of this study was to systematically review the correlation between osteoporotic vertebral fractures and BMD, and to evaluate the capacity of QCT and DXA to distinguish between osteoporosis with vertebral fracture and osteoporosis without fracture based on vBMD and aBMD.
Methods
Literature search
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and was registered at the International Prospective Register of Systematic Reviews (Number: CRD42022349185) [20]. The literature search was carried out in PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), WanFang Database, and China Science and Technology Journal Database up to 3 July, 2022, in both English and Chinese languages. The Medical Subject Headings (Mesh) and keywords used for retrieval are presented in the Online Resource by taking the form of PubMed as an example (Online Resource 1).
Inclusion and exclusion criteria
The included articles were selected by the following criteria: (a) the subjects were postmenopausal women or the elderly with osteoporosis; (b) the articles conducted both QCT and DXA tests and provided BMD; (c) the article provided clinical information related to occurrence of vertebral fracture.
The exclusion criteria were as follows: (a) duplicate publications; (b) reviews, letters, conference abstracts, posters, case reports, and meta-analyses; (c) articles with insufficient data or information about fracture sites.
Quality assessment
Two reviewers (Lin Chen and Xin-yi Wu) independently extracted the following data from qualified studies: first author’s last name, year of publication, sample size, age of subjects, scanning site, and bone parameters assessed by QCT and DXA.
The Newcastle–Ottawa scale (NOS) was used to evaluate the quality of articles using a star system based on nine questions. The criteria of NOS included the selection of study groups, comparability of the groups, and ascertainment of either the exposure or outcome of interest for case–control or cohort studies respectively [21]. Each study could obtain 0–9 stars, and a higher number of stars indicated a higher quality study.
Statistical analysis
The analysis for studying the ability of QCT and DXA to differentiate fracture was evaluated by Review Manager 5.4 (Nordic Cochrane Center, Copenhagen, Denmark) and RStudio (version 1.4.1106) with meta package, and the results were presented in terms of weighted mean difference (WMD) along with 95% confidence intervals (CIs). Heterogeneity was assessed by Higgins inconsistency index (I2) [22]. The randomized effect model was selected for combining effect indicators when the heterogeneity was significant (I2 values > 50%); otherwise, the fixed effect model was used. Sensitivity analyses were performed to test the robustness of the outcomes. Deeks’ funnel plot was employed to investigate the publication bias and was tested by Egger’s test.
Results
Literature search
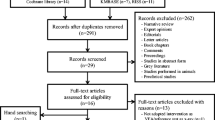
A total of 388 studies were initially identified from five databases (Fig. 1). After deleting duplicates, 300 studies were retained. After reviewing the titles and abstracts, 196 articles were excluded due to the age of subjects and article types. Among the remaining 104 studies, a comprehensive full-text review was conducted, leading to the exclusion of 98 studies. Ultimately, six articles were included in the analysis.
Quality of the studies assessed by Newcastle–Ottawa scale (NOS)
The quality of the studies was assessed by Newcastle–Ottawa Quality Assessment Scale (NOS), as shown in Table 1[23,24,25,26,27,28]. All six studies included in the analysis were categorized as case–control studies. Regarding the "Selection" criteria, two out of the six studies received two stars, while the remaining four studies received three stars, indicating a moderate overall quality for this aspect. The primary quality concerns in this category were related to the inadequate representation of the case cohort and the unsatisfactory selection of the control group. In terms of "Comparability", two studies got one star each, and the other four studies were given two stars. The rationale behind scoring one star was the limited control or clarification of factors beyond age, such as height and weight. Within the “Exposure” category, all six studies were assigned three stars, signifying a high level of quality in this aspect. In summary, the NOS assessment from the six included studies indicated a moderate overall quality.
Data extraction
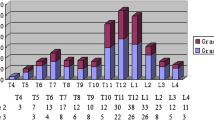
The outcomes of data extraction from the six studies, which employed QCT and DXA methods to assess BMD in fracture and non-fracture populations were summarized in Tables 2 and 3, respectively. A total of 610 individuals, ranging in age from 45 to 90 years, were included in this analysis. They were divided into two groups: osteoporosis with vertebral fractures in the “Fracture” and osteoporosis only in the “Non-fracture”. Among the results measured by QCT expressed in mg/cm3, the scanning sites of four studies were vertebrae and those of two studies were femur. As for DXA results reported in g/cm2, five studies scanned the vertebrae site and one scanned the femur site.
Heterogeneity assessment of measurements related to fracture risk meta-analysis
The overall heterogeneity in the six studies on fracture risk was low. This was determined through a range of I2 values, from 0% (P = 0.42) when assessed by aBMD, to 40% (P = 0.14) when assessed by vBMD. Moreover, after excluding studies one by one for analysis, no influential outliers were found. In summary, the findings from the six studies were not affected by heterogeneity.
Both vBMD and aBMD discriminate fracture status
There were significant differences in vBMD detected via QCT and aBMD detected through DXA between both the vertebral fracture group and the non-fracture group (P < 0.01). The WMD were − 27.08 (95%CI − 31.24 to − 22.92) for vBMD and − 0.05 (95%CI − 0.08 to − 0.03) for aBMD, as illustrated in Figs. 2 and 3. Hence, both vBMD and aBMD effectively differentiate the fracture status, but vBMD exhibits superior discriminatory ability.
Sensitivity analysis and publication bias
After excluding studies one by one and combining the results of other studies for sensitivity analysis, no significant effect was observed (Fig. 4 and Fig. 5). Two funnel plots were constructed—one for vBMD (Fig. 6) and another for aBMD (Fig. 7)—to assess the potential presence of publication bias. Asymmetry was tested by Egger’s test, yielding P-values of 0.5418 for vBMD and 0.3846 for aBMD. These results indicated that there was no publication bias across the six studies.
Discussion
How to “quantify” osteoporosis?
The structural integrity of bones is maintained through continuous remodeling in adulthood, in which osteoclasts absorb old or damaged bones and osteoblasts format new bones [29]. With advancing age, this process becomes imbalanced, with bone absorption exceeding formation, resulting in a loss of bone mass [30]. Cortical bone loss contributes to the enlargement of the bone marrow cavity, thinning of the cortex, and reduction in BMD. Trabecular bone loss, on the other hand, leads to damage of the trabecular structure, diminished trabecular thickness, and reduced connectivity [31]. This progression develops into the current definition of osteoporosis.
There is still no perfect definition of osteoporosis. The symptom of osteoporosis is not only the loss of some vertebrae or parts of bone, but also the general defect caused by bone degeneration with age, which leads to a high risk of fracture [32]. The present consensus for diagnosing osteoporosis is based on low BMD, which correlates with decreased loading strength of vertebral bodies and increased vertebral compression fracture risk. Previous studies have also proved the correlation between low BMD and an elevated risk of osteoporotic fractures [33]. Vertebral fractures, often regarded as a hallmark of osteoporosis, serve as significant indicators of compromised bone structure and diminished bone strength [34, 35].
Previous studies have documented that spinal BMD not only predicts the long-term risk of vertebral fractures, but also has a strong correlation with other types of fractures [16, 36, 37]. The drug intervention for the treatment of osteoporosis has a significantly higher response to the prevention of vertebral fractures than other fractures, which further emphasizes the unique role of vertebral fractures in osteoporosis [38]. Therefore, the spine may be an ideal site for BMD measurement in the diagnosis of osteoporosis, which has an important reference value for vertebral fracture and overall bone loss [35].
Application of DXA and QCT in the spine
aBMD measured by DXA as a reference standard in osteoporosis diagnosis
DXA measures the aBMD by obtaining the X-ray attenuation of patients exposed to low levels of X-ray radiation. The attenuation of each pixel in the predefined region of interest is summarized and converted to aBMD in units of g/cm2 using the previously established tissue quality attenuation coefficient [39, 40]. aBMD at the spine and hip is the reference standard for osteoporosis diagnosis. However, as our comprehension of bone microstructure advances, its significance in fracture risk prediction has encountered challenges [41, 42]. The prevalence of vertebral fractures varies across the vertebral level, with peaks in the middle of the spine (T7-T8) and the thoracolumbar junction (T12-L1). Vertebral fractures at these specific sites are more strongly associated with the risk of new fractures in the upper than in the lower spine [43]. It's important to note that DXA has certain limitations: it lacks accuracy in measuring BMD in the thoracic spine due to the influence of soft tissues and chest coverage [44]. Furthermore, DXA cannot provide detailed information about bone microstructure [45], consequently failing to adequately explain the correlation between aBMD and bone structure parameters [46]. The vertebral fracture assessment technique is an extension of DXA that attempts to evaluate vertebral fractures from a more comprehensive point of view, but certain limitations still exist, including its inability to identify vertebrae between T7-L4 and to detect common osteoporosis-related sclerosis or lytic changes [47].
vBMD measured by QCT in osteoporosis
vBMD measured by QCT requires calibrating the computed tomography values in Hounsfield units (HU) according to known density standards to calculate the equivalent density [48]. QCT can selectively measure trabecular or cortical BMD based on the region of interest, allowing further assessment of the parameters related to bone microstructure [49]. Moreover, it can accurately distinguish soft tissue compartments, minimizing the impact of extraosseous components [50]. A cross-sectional study found that QCT was more sensitive compared to DXA in detecting osteoporosis in postmenopausal women [51]. In a cohort study, it was found that QCT performed better in the risk assessment of imminent vertebral fractures than DXA [52].
Considering radiation exposure, it is advisable to minimize the number of vertebrae measured when utilizing QCT, with L1-L2 being the most commonly recommended site for evaluation [53]. According to the American Society of Radiology, the average BMD of L1-L2 is used for radiological diagnosis of osteopenia and osteoporosis [54]. A previous study has demonstrated high concordance between thoracic and lumbar BMD measured by QCT [55]. Another study also showed a strong correlation between the average BMD of L1–L2 and the other lumbosacral vertebrae (L3–S1) [56].
Due to disparities in equipment, imaging software, and the expertise of analysts, vBMD estimated by QCT is still individualized. Nevertheless, promising strides toward standardization have been highlighted through several multi-center clinical trials [48]. We look forward to an official recommendation to provide more details such as patient variables and calibration methods to guide clinical use of QCT in measuring vBMD.
In this study, we analyzed the ability of vBMD detected by QCT and aBMD detected by DXA in distinguishing between osteoporosis patients with vertebral fractures and those without fractures. Our findings indicated that both vBMD assessed by QCT and aBMD assessed by DXA can effectively differentiate osteoporotic fractures regardless of the scanning site, and vBMD (WMD = − 27.08; 95% CI − 31.24 to − 22.92) performed better than aBMD (WMD = − 0.05; 95% CI − 0.08 to − 0.03). Among the six articles, the scanned site of two of QCT [27, 28] and one of DXA were at the femur, and the others scanned more than two vertebrae between L1 and L4, which was consistent with the recommendation [13, 54]. Regrettably, the precise location of vertebral fractures was not described in detail in the six articles, thereby limiting us to deeply analyzing the correlation between BMD and vertebral fractured site. Interestingly, we found that even the BMD obtained from femur scans exhibited an association with vertebral fractures, suggesting that osteoporosis was an overall degeneration of bone health.
There are limitations needed to be improved in our research. In the analysis of evaluating the ability of QCT and DXA to distinguish fractures, it would be inappropriate to conclude that QCT is superior to DXA in identifying individuals with fractures based solely on vBMD and aBMD because we didn’t clear whether different units will lead to different results related to fracture risk. Also, the analysis of fracture risk based on BMD was still insufficient, and the quality of bone microstructure should be another important factor in fracture risk. However, we can deduce that osteoporosis associated with vertebral fracture risk may exhibit, on average, deficits of − 27.08 in vBMD and − 0.05 in aBMD, in comparison to osteoporosis without fracture. In the future, we may focus on patients with fractures and further study the application of QCT in osteoporotic fractures.
This paper demonstrated the strong correlation between BMD and osteoporotic fracture risk obtained by QCT and DXA, and identifies the superiority of QCT in predicting osteoporotic fracture risk.
Data availability
The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available in the article and supplementary information.
References
WA P (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94(6):646–650. https://doi.org/10.1016/0002-9343(93)90218-e
NIH Consensus Development Panel on Osteoporosis Prevention. Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795. https://doi.org/10.1001/jama.285.6.785
Sözen T, Özışık L, Başaran NÇ (2017) An overview and management of osteoporosis. Eur J Rheumatol 4(1):46–56. https://doi.org/10.5152/eurjrheum.2016.048
Glaser DL, Kaplan FS (1997) Osteoporosis. Definition and clinical presentation. Spine 22(24 Suppl):12S-16S. https://doi.org/10.1097/00007632-199712151-00003
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P (2016) Bone mechanical properties and changes with osteoporosis. Injury 47(Suppl 2):S11–S20. https://doi.org/10.1016/S0020-1383(16)47003-8
Cozadd AJ, Schroder LK, Switzer JA (2021) Fracture risk assessment: an update. J Bone Joint Surg 103(13):1238–1246. https://doi.org/10.2106/JBJS.20.01071
Lorentzon M, Cummings SR (2015) Osteoporosis: the evolution of a diagnosis. J Intern Med 277(6):650–661. https://doi.org/10.1111/joim.12369
Liu J, Curtis EM, Cooper C, Harvey NC (2019) State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest 42(10):1149–1164. https://doi.org/10.1007/s40618-019-01041-6
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
Yedavally-Yellayi S, Ho AM, Patalinghug EM (2019) Update on osteoporosis. Prim Care 46(1):175–190. https://doi.org/10.1016/j.pop.2018.10.014
Glüer CC (2017) 30years of DXA technology innovations. Bone 104:7–12. https://doi.org/10.1016/j.bone.2017.05.020
Burt LA, Manske SL, Hanley DA, Boyd SK (2018) Lower bone density, impaired microarchitecture, and strength predict future fragility fracture in postmenopausal women: 5-year follow-up of the calgary CaMos cohort. J Bone Mineral Res Off J Am Soc Bone Mineral Res 33(4):589–597. https://doi.org/10.1002/jbmr.3347
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group (1994) World Health Organization technical report series vol 843, pp 1–129
Armbrecht G, Felsenberg D, Ganswindt M, Lunt M, Kaptoge SK, Abendroth K, Aroso Dias A, Bhalla AK, Cannata Andia J, Dequeker J, Eastell R, Hoszowski K, Lyritis G, Masaryk P, van Meurs J, Miazgowski T, Nuti R, Poór G, Redlund-Johnell I, Reid DM, European Vertebral Osteoporosis Study and European Prospective Osteoporosis Study Groups (2017) Degenerative inter-vertebral disc disease osteochondrosis intervertebralis in Europe: prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Rheumatology 56(7):1189–1199. https://doi.org/10.1093/rheumatology/kex040
Schultz K, Wolf JM (2019) Emerging technologies in osteoporosis diagnosis. J Hand Surg 44(3):240–243. https://doi.org/10.1016/j.jhsa.2018.07.006
Van der Klift M, De Laet CE, McCloskey EV, Hofman A, Pols HA (2002) The incidence of vertebral fractures in men and women: the Rotterdam Study. J Bone Mineral Res Off J Am Soc Bone Mineral Res 17(6):1051–1056. https://doi.org/10.1359/jbmr.2002.17.6.1051
Link TM, Lang TF (2014) Axial QCT: clinical applications and new developments. J Clin Densitom Off J Int Soc Clin Densitom 17(4):438–448. https://doi.org/10.1016/j.jocd.2014.04.119
Engelke K (2017) Quantitative computed tomography-current status and new developments. J Clin Densitom Off J Int Soc Clin Densitom 20(3):309–321. https://doi.org/10.1016/j.jocd.2017.06.017
Mao SS, Li D, Syed YS, Gao Y, Luo Y, Flores F, Child J, Cervantes M, Kalantar-Zadeh K, Budoff MJ (2017) Thoracic quantitative computed tomography (QCT) can sensitively monitor bone mineral metabolism: comparison of thoracic QCT vs lumbar QCT and dual-energy X-ray absorptiometry in detection of age-relative change in bone mineral density. Acad Radiol 24(12):1582–1587. https://doi.org/10.1016/j.acra.2017.06.013
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2007) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp. Accessed on 23 July 2022
Higgins JPT, Thompson SG, Deeks J, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Cai YZ, Wang LY, Wu SY (2009) Lan JX (2009) Evaluation of bone mineral density measurement of lumbar vertebrae by volumetric quantitative CT in postmenopausal women (in Chinese). Chin J Radiol 2:119–125
Duboeuf F, Jergas M, Schott AM, Wu CY, Glüer CC, Genant HK (1995) A comparison of bone densitometry measurements of the central skeleton in post-menopausal women with and without vertebral fracture. Br J Radiol 68(811):747–753. https://doi.org/10.1259/0007-1285-68-811-747
Jergas M, Breitenseher M, Glüer CC, Yu W, Genant HK (1995) Estimates of volumetric bone density from projectional measurements improve the discriminatory capability of dual X-ray absorptiometry. J Bone Mineral Res Off J Am Soc Bone Mineral Res 10(7):1101–1110. https://doi.org/10.1002/jbmr.5650100715
Rehman Q, Lang T, Modin G, Lane NE (2002) Quantitative computed tomography of the lumbar spine, not dual x-ray absorptiometry, is an independent predictor of prevalent vertebral fractures in postmenopausal women with osteopenia receiving long-term glucocorticoid and hormone-replacement therapy. Arthritis Rheum 46(5):1292–1297. https://doi.org/10.1002/art.10277
Wu SY, Qi J, Wang B et al (2005) Quantitative study on proximal femoral of osteoporotic elderly women with 16-slice CT (in Chinese). J Med Imaging 15(10):885–889. https://doi.org/10.3969/j.issn.1006-9011.2005.10.020
Wu SY, Jia HH, Hans D, Lan J, Wang LY, Li JX, Cai YZ (2009) Assessment of volumetric bone mineral density of the femoral neck in postmenopausal women with and without vertebral fractures using quantitative multi-slice CT. J Zhejiang Univ Sci B 10(7):499–504. https://doi.org/10.1631/jzus.B0820409
Gao Y, Patil S, Jia J (2021) The development of molecular biology of osteoporosis. Int J Mol Sci 22(15):8182. https://doi.org/10.3390/ijms22158182
Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA (2014) Bone quality: the determinants of bone strength and fragility. Sports Med 44(1):37–53. https://doi.org/10.1007/s40279-013-0100-7
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393(10169):364–376. https://doi.org/10.1016/S0140-6736(18)32112-3
Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L, Kilgore M, Saag K (2011) Which fractures are most attributable to osteoporosis? J Clin Epidemiol 64(1):46–53. https://doi.org/10.1016/j.jclinepi.2010.07.007
Cummings SR, Bates D, Black DM (2002) Clinical use of bone densitometry: scientific review. JAMA 288(15):1889–1897. https://doi.org/10.1001/jama.288.15.1889
Ettinger B, Black DM, Nevitt MC, Rundle AC, Cauley JA, Cummings SR, Genant HK (1992) Contribution of vertebral deformities to chronic back pain and disability. The study of osteoporotic fractures research group. J Bone Mineral Res Off J Am Soc Bone Mineral Res 7(4):449–456. https://doi.org/10.1002/jbmr.5650070413
Melton LJ 3rd, Riggs BL, Keaveny TM, Achenbach SJ, Kopperdahl D, Camp JJ, Rouleau PA, Amin S, Atkinson EJ, Robb RA, Therneau TM, Khosla S (2010) Relation of vertebral deformities to bone density, structure, and strength. J Bone Mineral Res Off J Am Soc Bone Mineral Res 25(9):1922–1930. https://doi.org/10.1002/jbmr.150
Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, Nevitt MC, Cummings SR (2007) Long-term risk of incident vertebral fractures. JAMA 298(23):2761–2767. https://doi.org/10.1001/jama.298.23.2761
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR, Osteoporotic Fractures Research Group (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Mineral Res Off J Am Soc Bone Mineral Res 18(11):1947–1954. https://doi.org/10.1359/jbmr.2003.18.11.1947
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, HORIZON Pivotal Fracture Trial (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822. https://doi.org/10.1056/NEJMoa067312
Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, Zemel BS, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S (2008) International society for clinical densitometry 2007 adult and pediatric official positions. Bone 43(6):1115–1121. https://doi.org/10.1016/j.bone.2008.08.106
Pennington Z, Ehresman J, Lubelski D, Cottrill E, Schilling A, Ahmed AK, Feghali J, Witham TF, Sciubba DM (2021) Assessing underlying bone quality in spine surgery patients: a narrative review of dual-energy X-ray absorptiometry (DXA) and alternatives. Spine J Off J N Am Spine Soc 21(2):321–331. https://doi.org/10.1016/j.spinee.2020.08.020
Williams S, Khan L, Licata AA (2021) DXA and clinical challenges of fracture risk assessment in primary care. Clevel Clin J Med 88(11):615–622. https://doi.org/10.3949/ccjm.88a.20199
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Pignone M, Silverstein M, Simon MA, Tseng CW, Wong JB (2018) Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. JAMA 319(24):2521–2531. https://doi.org/10.1001/jama.2018.7498
Nevitt MC, Ross PD, Palermo L, Musliner T, Genant HK, Thompson DE (1999) Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. The Fracture Intervention Trial Research Group. Bone 25(5):613–619. https://doi.org/10.1016/s8756-3282(99)00202-1
Bolotin HH (2007) DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone 41(1):138–154. https://doi.org/10.1016/j.bone.2007.02.022
Crabtree N, Ward K (2015) Bone densitometry: current status and future perspective. Endocr Dev 28:72–83. https://doi.org/10.1159/000380994
Cosman F, Schnitzer MB, McCann PD, Parisien MV, Dempster DW, Lindsay R (1992) Relationships between quantitative histological measurements and noninvasive assessments of bone mass. Bone 13(3):237–242. https://doi.org/10.1016/8756-3282(92)90203-9
Zeytinoglu M, Jain RK, Vokes TJ (2017) Vertebral fracture assessment: enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone 104:54–65. https://doi.org/10.1016/j.bone.2017.03.004
Brunnquell CL, Winsor C, Aaltonen HL, Telfer S (2021) Sources of error in bone mineral density estimates from quantitative CT. Eur J Radiol 144:110001. https://doi.org/10.1016/j.ejrad.2021.110001
Samelson EJ, Christiansen BA, Demissie S, Broe KE, Louie-Gao Q, Cupples LA, Roberts BJ, Manoharam R, D’Agostino J, Lang T, Kiel DP, Bouxsein ML (2012) QCT measures of bone strength at the thoracic and lumbar spine: the Framingham Study. J Bone Mineral Res Off J Am Soc Bone Mineral Res 27(3):654–663. https://doi.org/10.1002/jbmr.1482
Yu EW, Thomas BJ, Brown JK, Finkelstein JS (2012) Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone mineral Res Off J Am Soc Bone Mineral Res 27(1):119–124. https://doi.org/10.1002/jbmr.506
Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W (2013) Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Int J Endocrinol. https://doi.org/10.1155/2013/895474
Löffler MT, Jacob A, Valentinitsch A, Rienmüller A, Zimmer C, Ryang YM, Baum T, Kirschke JS (2019) Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur Radiol 29(9):4980–4989. https://doi.org/10.1007/s00330-019-06018-w
Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM (2008) Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom Off J Int Soc Clin Densitom 11(1):123–162. https://doi.org/10.1016/j.jocd.2007.12.010
American College of Radiology (2018) ACR–SPR–SSR PRACTICE PARAMETER FOR THE PERFORMANCE OF MUSCULOSKELETAL QUANTITATIVE COMPUTED TOMOGRAPHY (QCT). https://www.acr.org/-/media/ACR/Files/Practice-Parameters/QCT.pdf. Accessed July 24 2022
Budoff MJ, Khairallah W, Li D, Gao YL, Ismaeel H, Flores F, Child J, Carson S, Mao SS (2012) Trabecular bone mineral density measurement using thoracic and lumbar quantitative computed tomography. Acad Radiol 19(2):179–183. https://doi.org/10.1016/j.acra.2011.10.006
Salzmann SN, Shirahata T, Yang J, Miller CO, Carlson BB, Rentenberger C, Carrino JA, Shue J, Sama AA, Cammisa FP, Girardi FP, Hughes AP (2019) Regional bone mineral density differences measured by quantitative computed tomography: does the standard clinically used L1–L2 average correlate with the entire lumbosacral spine? Spine J Off J N Am Spine Soc 19(4):695–702. https://doi.org/10.1016/j.spinee.2018.10.007
Acknowledgements
Not applicable.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (No. 2022-NHLHCRF-LX-02–0108).
Author information
Authors and Affiliations
Contributions
LC and XW contributed equally to this work and share first authorship. XM and LC provided the concept and designed the study. LC and XW conducted data analysis. LC and XW wrote the original manuscript. All authors contributed to reviewing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Lin Chen, Xin-yi Wu, Qi Jin, Guang-yao Chen and Xiao Ma declare that they have no conflict of interest.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L., Wu, Xy., Jin, Q. et al. The correlation between osteoporotic vertebrae fracture risk and bone mineral density measured by quantitative computed tomography and dual energy X-ray absorptiometry: a systematic review and meta-analysis. Eur Spine J 32, 3875–3884 (2023). https://doi.org/10.1007/s00586-023-07917-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07917-9