Abstract
Objectives
No evidence-based treatment exists for adult spinal deformity (ASD) patients with chronic low back pain (CLBP). Aim of this study: evaluate a combined physical and psychological programme (CPPP) for ASD patients with CLBP and to compare this with a non-ASD-cohort with CLBP.
Methods
Data were extracted from the database of CLBP-patients for whom surgery is not an option and completed CPPP. Two cohorts were selected: an ASD-cohort (n = 80) based on a Cobb angle of > 10° and a consecutive age- and gender-matched non-ASD-cohort (n = 240). Primary outcome: functional status (Oswestry Disability Index; ODI). Secondary outcomes: pain intensity, self-efficacy and quality of life. Assessments: pre and post treatment, one-month and one-year follow-up (FU). Clinical relevance: minimal important clinical change (MCIC; ODI 10 points), patient acceptable symptom state (PASS; ODI ≤ 22).
Results
Demographics ASD-cohort: 79% female, mean age 50.9 (± 14.1) years, mean CLBP duration 15.5 (± 12.5) years, mean Cobb angle 21.4 (± 9.4)°. Non-ASD-cohort: not significantly different. Both cohorts improved in functional status (F[1,318] = 142.982, p < .001; r = 0.31). The ASD-cohort improved from mean ODI 39.5(± 12.0) at baseline to mean ODI 31.8(± 16.5) at one-year FU. Clinical relevance: 51% of the ASD patients reached MCIC and 33% reached a PASS. An interaction effect is shown between time and both cohorts (F[1,318] = 8.2, p = .004; r = 0.03); however, not clinically relevant. All secondary outcomes: improvement at one-year FU.
Conclusion
This is the first study showing beneficial outcomes of a non-surgical treatment in selected ASD patients with longstanding CLBP. Improvement is shown in functional status, and appeared equivalent to the non-ASD cohort.
Level of Evidence 1
Diagnostic: individual cross-sectional studies with the consistently applied reference standard and blinding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult spinal deformity (ASD) has a high prevalence in individuals aged older than 60 years, affecting up to 68% of that population [1]. The clinical and economic burden of ASD for society is growing [2, 3]. The ASD spectrum includes primary degenerative scoliosis (de novo degenerative lumbar scoliosis [DNDLS]), progressive adolescent idiopathic scoliosis in adulthood (AdIS) and secondary adult deformity (e.g. hyperkyphosis, iatrogenic sagittal deformity, focal deformity due to multiple degenerative disc disease with global deformity, and post-traumatic spinal deformity) [2, 4]. A common symptom in spinal deformity is back pain [5].
ASD can be managed non-surgically or surgically. Non-surgical management is regarded as the first line treatment, despite an absence of strong supporting evidence [2, 6]. This is underlined in the systematic review of Teles et al. [6]. The authors included five studies (545 patients) of non-surgical care in ASD and showed that the best existing evidence is derived from observational studies. A high risk of bias was shown, mainly because the indications and protocols for treatment were not consistent in any of the evaluated studies. Quantitative analyses of non-surgical cohorts did not demonstrate significant changes in quality of life in patients after 2 years observation. The authors concluded that, as yet, no evidence-based alternative exists for surgical care for patients with ASD [6].
Although for patients with back pain a plethora of non-invasive treatments exists [7], many authors have emphasised the biopsychosocial influences on the development of chronic low back pain (CLBP) and persistence of symptoms [8] and a broad multidimensional approach is widely recognised.
Evidence exists for a combined physical and psychological programme (CPPP) in patients with CLBP that uses a multidimensional approach and which is based on cognitive behavioural principles. Clinically relevant improvements were shown in daily functioning, pain and quality of life. Positive one year results and reduction in healthcare utilization are maintained at a minimum of 5 year follow up [9, 10]. The question remains whether such a multimodal programme is beneficial for ASD patients as well.
The aim of this study is to evaluate the performance of a CPPP for ASD patients who have CLBP and to compare the treatment outcomes with non-ASD patients (CLBP patients without ASD).
Materials and methods
Study design and setting
The study is a retrospective analysis of a historical comparative cohort and follows the STROBE guidelines [11]. The data of this comparative cohort study were prospectively collected in a mono-centre outcomes registry for consecutive patients with CLBP. For this study, the data of an historical cohort of patients with CLBP and diagnosed with ASD were compared with a symptomatic non-ASD cohort.
The study was over a five year period; data were recorded in the period April 2014–September 2019.
The study protocol was approved by the institution’s internal investigational review board. Exemption for ethical approval was obtained by the medical ethical committee of the Radboud University Nijmegen Medical Centre, as the Dutch Act on Medical Research involving Human Subjects does not apply to screening questionnaires that are part of routine clinical practice. All patients had the opportunity to declare that their anonymized data were not used for other purposes than scientific research. For this study, fully anonymized and de-identified data were obtained for analysis and report.
Participants
The data used in this study were collected from patients with CLBP who were referred to the CPPP by spine surgeons at a tertiary orthopaedic hospital, specialized in spinal care. Continuous outcome monitoring, using patient-reported outcome measures (PROMs) is part of the programme (Nijmegen Decision Tool for CLBP [12]). Patients were eligible for referral if conservative treatment in primary care had failed and if there were no indications for surgery or invasive pain treatment. The main inclusion criteria for the programme were: CLBP for at least six months, no medical restrictions on activity, age between 18 and 70 years, willingness to change behaviour and fluent in Dutch. Exclusion criteria were: involvement in litigation and/or compensation claims, and primary psychiatric disorders. The final inclusion for the programme was based on an assessment by a multidisciplinary team, consisting of a psychologist and a physiotherapist or psychomotor therapist.
From the data collected of this study group two cohorts were selected. Inclusion criteria for the ASD cohort: data were used of patients referred with CLBP and diagnosed with ASD, with an angular (Cobb) value of more than 10° in the coronal plane [4], and who completed the CPPP (i.e. including one-year follow-up assessment).
Inclusion criteria for the non-ASD cohort: data were used of patients referred with CLBP, with no diagnosed ASD, and who completed the CPPP (i.e. including one-year follow-up assessment). To compare the results of both cohorts, this cohort was matched in a 1:3 ratio, based on age (categories: ≤ 20, 21–40, 41–60, ≥ 61) and gender (male/female). The cohort was randomly ordered per category via the function RAND in Excel. The following selection in the file was drawn consecutively.
To verify the diagnosis of ASD as described in the patients’ referral letter, plain standing full spine radiographs of the lumbar spine were used. The Cobb angle of the lumbar spine was measured using IntelliSpace PACS (Philip) by one experienced orthopaedic spine surgeon. A Cobb angle of > 10 degrees was classified as ASD [4].
From the matched non-ASD group a random sample of 51 patients was selected to exclude ASD on plain radiographs.
Treatment/intervention
The intervention was a CPPP, based on cognitive behavioural principles, for patients with CLBP and previously described [9]. The CPPP was NICE CLBP guidelines compliant [13] and follows a standardized and predefined protocol. It was an interdisciplinary biopsychosocial programme consisting of an intensive two week residential group treatment, followed by a one-month (FU1) and one-year follow-up (FU2). The interdisciplinary team consisted of a psychologist, a physiotherapist, and a psychomotor therapist who were extensively educated in cognitive behavioural techniques for chronic pain. The main aim of the intervention was to improve daily functioning through behavioural change. This was achieved by increasing the capability to self-manage the CLBP.
Data collected
Patient characteristics and the data from online self-report questionnaires regarding functional status, back pain intensity, self-efficacy and quality of life, were obtained at baseline assessment, at post-treatment (day 10; D10), at FU1, and at FU2 assessments, and were extracted from the institution’s patient outcome registry. All these self-report measures have previously been validated in CLBP samples [14,15,16]. The assessments were an integral part of the programme. The FU1 and FU2 were linked to face-to-face group sessions which minimizes potential non-response to questionnaires. At baseline assessment, participants provided information on age, gender, health status, pain history, and employment status (patient characteristics).
Outcome measures
Primary outcome measure
Functional status – Oswestry disability index (ODI version 2.1a; ODI)
The ODI is one of the principal condition-specific outcome measures used in the management of spinal disorders [17]. In this study, a Dutch version of ODI version 2.1a is used [18]. The ODI measures the impact of LBP on daily functioning in ten domains of daily life (disability). The total (sum) scores ranges between 0 and 100, with zero corresponding to ‘no disability’ and 100 representing the maximum disability possible, ‘bed-bound’. The minimal clinical important change (MCIC) and the minimal clinical important difference (MCID) as a measure for clinical relevance in this study is 10 points [14]. An ODI score ≤ 22 indicates the achievement of a patients acceptable symptom state (PASS) [19].
Secondary outcome measure
Back pain intensity – numeric pain rating scale (NPRS)
The pain intensity is measured on a six-item questionnaire in terms of severity and disturbance of daily activities on a scale between 0 and 100, with higher values indicating higher pain intensity and disturbance. The MCIC for clinical relevance in this study is considered to be a reduction of 20 points, or 30% [14].
Pain Self-efficacy Questionnaire– PSEQ
The PSEQ is a 10-item inventory that measures the strength of the patient’s beliefs about their ability to accomplish a range of activities despite the pain. Belief in self-efficacy influences the possibility of effectively using pain-coping strategies. The measure is used to evaluate the participants’ ability to self-manage their pain complaints. A Dutch translation is used. The total score ranges from 0 to 60, with higher scores indicating higher perceived self-efficacy beliefs [20]. For patients with CLBP the MCIC for the PSEQ is 5.5 points (9% of the scale range) [15].
Quality of Life – MOS-short Form 36 (SF36-MCS and SF36-PCS)
The SF36 [21] is a generic questionnaire to measure health-related quality of life. The validated Dutch language version has been used [22]. The questionnaire contains 36 items divided in eight subscales. The subscales results are recombined into two summery scores: the SF36 Physical Component Score (SF36 PCS) and the SF36 Mental health Component Score (SF36 MCS). The total score ranges from 0 to 100, with a higher score indicating better quality of life. In patients with subacute and chronic LBP, improvements > 3.77 in MCS and > 3.29 in PCS, can be considered clinically relevant [16].
Statistical analysis
Data were checked for normality using the Shapiro–Wilk test. All variables were normally distributed. Descriptive statistics were used to describe categorical (number and percentages) and continuous variables (means and standard deviations [SD]) for the ASD cohort and matched non-ASD cohort. Baseline characteristics of both cohorts were compared using unpaired t-test for continuous parameters and X2-test for categorical parameters. To evaluate primary outcomes over time the ASD cohort was compared with the matched non-ASD cohort. Repeated measures analysis of variance (RM-ANOVA) was used to evaluate outcomes over time and to determine between group differences on treatment outcome (ASD-cohort versus matched non-ASD cohort). For secondary outcomes RM-ANOVA was used to evaluate the ASD-cohort over time. A p < 0.05 was considered statistically significant. Statistical analyses were conducted in STATA (version 14.0 for Windows; StataCorp, College Station, Texas, USA).
Results
Participant selection procedure
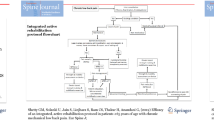
The participant selection procedure is shown in Fig. 1.
Flowchart participant selection procedure. *Of the ASD cohort 10 patients were not included because they did not complete the CPPP. 4 Patients didn’t complete the programme. Other treatment was preferred (for example individual revalidation, surgery abroad). 6 Patients didn’t fill in the FU2 questionnaire, because of technical problems or life events (death or illness/surgery of a spouse). ASD = chronic low back pain patients with adult spinal deformity; non-ASD = chronic low back pain patients without adult spinal deformity, FU2: one-year follow-up assessment
Baseline patient characteristics (Table 1)
The baseline characteristics of the ASD cohort and non-ASD cohort are shown in Table 1. The two cohorts are equivalent at baseline (p > 0.05).
Primary outcome: functional status (Table 2 and 3, Fig. 2)
The ASD cohort improved from mean ODI 39.5(± 12.0) at baseline to mean ODI 31.8(± 16.5) at FU2. Both cohorts improved fast in functional status and maintenance of results at FU2 is shown (F[1,318] = 142.982, p < 0.001; r = 0.31; Fig. 2).
An interaction effect is shown between time (baseline and FU2) and both cohorts (F[1,318] = 8.2, p = 0.004; r = 0.03); however, this effect is not clinically relevant. The mean difference at FU2 is 4.1 points (< 10 points MCID).
Secondary outcome (Tables 2 and 3)
Back pain intensity – numeric pain rating scale (NPRS)
The mean NPRS of the ASD cohort improved from 58.4(± 19.1) to 42.1(± 28.3). This a significant improvement (F[1,272] = 101.287, p < 0.001; r = 0.271).
Quality of life – MOS-short form 36 (SF36-MCS and SF36-PCS).
The mean physical component of quality of life improved from 39.8(± 14.4) to 56.6(± 20.3) and the mean mental health component improved from 55.7(± 18.3) to 67.2(± 20.1). These are significant improvements for both the physical component (F[1,318] = 180.525, p < 0.001; r = 0.362) and the mental component (F[1,318] = 61.932, p < 0.001; r = 0.163).
Pain self-efficacy questionnaire– PSEQ (Dutch translation)
Mean self-efficacy improved from 30.1(± 10.5) to 43.4(± 13.3). This is a significant improvement (F[1,316] = 291.664, p < 0.001; r = 0.480).
Clinical relevance for both primary as secondary outcome measures are shown in Table 3.
Discussion
The aim of this study was to evaluate the performance of a combined physical and psychological programme (CPPP) in patients with adult spinal deformity (ASD) who have chronic low back pain (CLBP); the ASD cohort, and to compare the treatment outcomes with CLBP patients without ASD; the non-ASD cohort.
The results of this study showed that the ASD cohort improved fast and their clinically relevant improvements are maintained for at least one year. The improved outcomes appeared clinically equivalent to the non-ASD cohort; there are no statistical differences between the two cohorts with respect to any of the outcome parameters at any time-point of follow-up. This is surprising given the assumption that the ASD cohort is in many respects a more complicated patient group to get a treatment effect on. It is also a unique finding, given the current literature on ASD, in which quantitative analyses of nonsurgical treatments is not able to demonstrate sustained changes in the quality of life of ASD patients [6].
It seems that the CPPP provides ASD patients effective tools to self-manage the CLBP in order to improve their quality of life both physically and mentally in the long run. This is supported by the improvements seen in self-efficacy, which is suggested to be an important factor that has implications for functional status. The systematic review of Jackson et al. [23], including 15,616 chronic pain patients (83 studies), demonstrated that self-efficacy facilitates recovery with lower levels of disability and pain. This has been supported by the study of van Hooff et al. [24], including 524 patients with CLBP, showing targeting self-efficacy in a CPPP is an important driver to fast improvement of functional status, and that patients are able to learn and apply pain self-management principles in a short period of time which is maintained for at least five years [10].
Although not clinically relevant, a significant interaction effect between time (baseline and one-year follow-up) and both cohorts is shown in functional status. Where improvement in the ASD cohort seems to stabilise after one-month follow-up, the improvement in the non-ASD cohort shows a trend of further improvement. Furthermore, all secondary outcomes (i.e. pain intensity, health-related quality of life [HR-QoL] and self-efficacy) showed maintenance of improvement over time, which was comparable to the non-ASD cohort. As such the CPPP seems a promising intervention. Although the improvement in functional status in the ASD cohort seems to stabilise, it is unlikely that this could be explained by the natural history of ASD. Whereas curve progression can occur over a mean follow-up of five years [25], we do not expect that there is significant curve progression at one-year follow-up in the ASD cohort.
Strengths and limitations
A strength of this study is the relatively large sample size of 80 patients with ASD. The authors are not aware of any previously published study that included a patient sample of this size. Furthermore, patient characteristics are described in detail. Other strengths of this study are that clear inclusion criteria exists for the CPPP and the CPPP follows a standardized and predefined protocol. This in contrast to any of the evaluated studies that Teles et al. included in their systematic review concerning conservative treatment in ASD [6]. By applying these clear inclusion criteria and using standardized and predefined treatment protocol, we tried to minimize the usual limitations in generalizability of a single centre study.
A complete case analysis was performed. This can be considered as a limitation. Although the number of patients who failed to complete the programme and the non-response on questionnaires at FU2 is relatively low (11% [10/90] ASD-cohort; 13% [158/1215] non-ASD cohort), this might bias the study results towards a positive outcome. By using a matching procedure for the non-ASD cohort with the same criteria as the ASD cohort the comparisons between the ASD and the non-ASD cohorts are valid.
In conclusion, this study is the first to show that a CPPP is beneficial, in functioning, self-efficacy, pain and HR-QoL for patients with ASD and with longstanding CLBP, for whom surgery is not an option. The ASD cohort and the non-ASD cohort behave in a similar manner to the treatment protocol. The clinically relevant improvements are maintained at one-year follow-up, and are meaningful for patients. At one-year follow-up assessment a third of the ASD patients have reached an acceptable symptom state with respect to function. In the future, this might suggest that healthcare utilization and health care costs could be diminished for this patient group.
References
Schwab F, Dubey A, Gamez L, El Fegoun AB, Hwang K, Pagala M, et al (2005) Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 30:1082–1085
Diebo BG, Shah NV, Boachie-Adjei O et al (2019) Adult spinal deformity-seminar. Lancet 394:160–172
McCarthy I, Hostin R, O’Brien M, Saigal R, Ames CP (2013) Health economic analysis of adult deformity surgery. Neurosurg Clin N Am 24:293–304
Aebi M (2005) The adult scoliosis. Eur Spine J 14:925–948
Théroux J, Stomski N, Hodgetts CJ, Ballard A, Khadra C, Le May S, Labelle H (2017) Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Therap. https://doi.org/10.1186/s12998-017-0143-1
Teles AR, Mattei TA, Righesso O, Falavigna A (2017) Effectiveness of operative and nonoperative care for adult spinal deformity: systematic review of the literature. Global Spine J 7:170–178
Haldeman S, Dagenais S (2008) A supermarket approach to the evidence-informed management of chronic low back pain. Spine J 8(1):1–7
Weiner BK (2008) Spine update: the biopsychosocial model and spine care. Spine (Phila Pa 1976) 33:219–223
van Hooff ML, van der Merwe JD, O’Dowd J, Pavlov PW, Spruit M, de Kleuver M, van Limbeek J (2010) Daily functioning and self-managment in patients with chronic low back pain after an intensive cognitive behavioural programme for pain management. Eur Spine J 19:1517–1526. https://doi.org/10.1007/s00586-010-1435-5
Groot D, van Hooff ML, Kroeze RJ, Monshouwer M, O’Dowd J, Horsting P, Spruit M (2019) Long-term results of an intensive cognitive behavioural pain management program for patients with chronic low back pain: a concise report of an extended cohort with a minimum of 5 year follow-up. Eur Spine J 28(7):1579–1585. https://doi.org/10.1007/s00586-019-05967-6
Vandenbroucke JP, von Elm E, Altman DG et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 4(10):1628–1654
Van Hooff ML, van Dongen JM, Coupé VM, Spruit M, Ostelo RWJG, de Kleuver M (2019) Can patient-reported profiles avoid unnecessary referral to a spine surgeon? An observational study to further develop the Nijmegen Decision Tool for chronic low back pain. PLoS One 13(9):e0203518
NICE National Institute for Health and Clinical Excellence (2016) Low back pain and sciatica in Over 16s: assessment and management. NICE guideline (NG59); London
Ostelo RW, Deyo RA, Stratford P et al (2008) Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 33:90–94
Chiarotto A, Vanti C, Cedraschi C, Ferrari S, de Lima E, SàResende F, Ostelo RW, Pillastrini P (2016) Responsiveness and minimal important change of the pain self-efficacy questionnaire and short forms in patients with chronic low back pain. J Pain 17(6):707–718
Díaz-Arribas MJ, Fernández-Serrano M, Royuela A, Kovacs FM, Gallego-Izquierdo T, Ramos-Sánchez M, Llorca-Palomera R, Pardo-Hervás P, Martín-Pariente OS (2017) Minimal clinically important difference in quality of life for patients with low back pain. Spine (Phila Pa 1976) 42(24):1908–1916
Fairbank JC, Pynsent PB (2000) The oswestry disability index. Spine (Phila Pa 1976) 25(22):2940–2952
Van Hooff ML, Spruit M, Fairbank JC, van Limbeek J, Jacobs WC (2015) The oswestry disability index (version 2.1a): validation of a Dutch language version. Spine (Phila Pa 1976) 40(2):E83–90
Van Hooff ML, Mannion AF, Staub LP, Ostelo RWJG, Fairbank JCT (2016) Determination of the Oswestry Disability Index score equivalent to a “satisfactory symptom state” in patients undergoing surgery for degenerative disorders of the lumbar spine. Spine J 16(10):1221–1230
Nicholas MK (2007) The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 11:153–163
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E (1998) Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 51:1055–1068
Jackson T, Wang Y, Wang Y, Fan H (2014) Self-efficacy and chronic pain outcomes: a meta-analytic review. J Pain 15: 800–814. https://doi.org/10.1016/j.jpain.2014.05.002
Van Hooff ML, Vriezekolk JE, Kroeze RJ, O’Dowd JK, van Limbeek J, Spruit M (2021) Targeting self-efficacy more important than dysfunctional behavioral cognitions in patients with longstanding chronic low back pain; a longitudinal study. Abstract Global Spine Congres 2021 (to be published Global Spine Journal)
Faraj S, te Hennese N, van Hooff M, Pouw M, de Kleuver M, Spruit M (2020) The natural history of progression in adult spinal deformity: a radiographic analysis. Global Spine J 10(3):272–279
Acknowledgements
The authors thank all patients and the multidisciplinary team at RealHealthNL, who were responsible for the assessments of the participants in the CPP programme, and Marieke Monshouwer, who read all 1322 referral letters.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoevenaars, E.H.W., Beekhuizen, M., O’Dowd, J. et al. Non-surgical treatment for adult spinal deformity: results of an intensive combined physical and psychological programme for patients with adult spinal deformity and chronic low back pain—a treatment-based cohort study. Eur Spine J 31, 1189–1196 (2022). https://doi.org/10.1007/s00586-022-07156-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07156-4