Abstract
Purpose
A multi-morbidity perspective of troublesome low back pain (LBP) has been highlighted for example in relation to respiratory disorders. Our purpose was to investigate whether respiratory disorders are risk factors for reporting troublesome LBP in people with no or occasional LBP at baseline.
Methods
This prospective cohort study was based on the Stockholm Public Health Cohort 2006/2010. We included adults reporting no or occasional LBP the last 6 months at baseline (n = 17,177). Exposures were self-reported asthma and/or Chronic Obstructive Pulmonary Disease (COPD). Outcome was troublesome LBP defined as reporting LBP a couple of days per week or more often that restricted work capacity or hindered daily activities to some or to a high degree, the last 6 months. Binomial regression models were used to calculate risk ratios (RR) with 95% confidence intervals (95% CI).
Results
Adjusted results indicate that those suffering from asthma had a risk of troublesome LBP at follow-up (RR 1.29, 95% CI 0.92–1.81) as do those suffering from COPD (RR 2.0, 95% CI 1.13–3.56). If suffering from asthma and concurrent COPD the RR was 3.55 (95% CI 1.58–7.98).
Conclusion
Our findings indicate that suffering from asthma and/or COPD increases the risk of developing troublesome LBP, which highlights the importance to consider the overall health of people at risk of troublesome LBP and to take the multi-morbidity perspective into consideration. Future longitudinal studies are needed to confirm our findings.
Graphic abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Low back pain (LBP) is one of the largest health problems for public healthcare systems all over the world and is rated first among 291 disorders with most years lived with disability [1,2,3]. The incidence of LBP is increasing alongside with a growing aging population [3]. The highest prevalence is reported in females and in those aged 40–80 years [2].
Low back pain has been previously discussed to be an own entity and not associated with other disorders [4]. The risk of developing LBP is proposed to be affected by several factors such as lumbar disk degeneration [5, 6], bio-mechanical [7], psychosocial and psychological [8], and work-related factors [5]. In addition, other risk factors, such as life style [9] and genetic influences [7, 10], for developing troublesome LBP are reported. Moreover, a multi-morbidity perspective of LBP in relation to other disorders has been highlighted, for example, in relation to osteoarthritis [11] and respiratory disorders (RD) [12]. The comorbidity perspective of LBP is relatively underinvestigated even if associations are reported [4, 11]. As such, the causality of the disorders is unclear and is suggested as the classic hen and egg situation of which comes first. Beeckmans et al. [12] stated that future research is needed to determine whether an association between LBP and RD is causative or not, to develop troublesome back pain.
Several cross-sectional studies report an association between disabling LBP and respiratory symptoms [11,12,13]. In 2003, Hestbaek et al. [14] reviewed the literature and reported an association between LBP and among others RD. A recent review reported associations between LBP and RD such as dyspnea and asthma [12]. Further, Hestbaek et al. [15, 16] did report an association between LBP and asthma in a younger cohort suggesting that the susceptibility for suffering from several disorders at younger age might reflect factors that are psychosocial and socioeconomic. In addition to such factors explaining comorbidity with LBP, physical factors are suggested. The diaphragm and other important breathing muscles may play a role in both postural control [17] and in spinal stiffness [18]. Among RD, chronic obstructive pulmonary disease (COPD) is a chronic condition that leads to a pathologic degeneration of the respiratory system [19,20,21]. Jansen’s et al. [22] studied people suffering from COPD and found that those with inspiratory muscle weakness showed a decreased reliance on back muscle proprioceptive signals during a balance task thus resulting in a decreased postural stability compared to healthy controls. In people with asthma hyperventilation is a problem affecting the breathing muscles [23].
As the evidence on the importance of RD to develop LBP is sparse, new knowledge may provide insights and thus clinical recommendation and management for these patients. Our aim was to investigate whether RD reported at baseline are risk factors for reporting troublesome LBP 4 years later in people with no or occasional LBP at baseline.
Methods
Study design
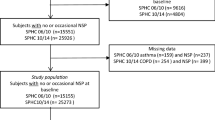
This prospective cohort study is based on the Stockholm Public Health Cohort (SPHC), 2006/2010 (n = 25,167), a population-based cohort setup by the Stockholm County Council to collect information about the factors and consequences of significant contributors to the burden of disease [24]. Details of the data collection are reported elsewhere [25]. The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (2015/1204–32).
Study population
We included adults ( > 18 years old) who answered the SPHC survey 2006/ 2010. The inclusion was based on a question in the SPHC survey: “During the previous six months, have you experienced low back pain?” (“No”, “Yes, a couple of days in the last six months”, “Yes, a couple of days each month, “Yes, a couple of days each week” and “Yes, everyday”). We included those who answered with either “No” or “Yes, a couple of days last six months” at baseline, here defined as no or occasional LBP. Those included with missing data on the questions on asthma and COPD (n = 176) were excluded from the cohort. Thus, our study population was n = 17,177 (Fig. 1).
People who reported LBP more often than occasional LBP at baseline were excluded (n = 7814). Among the excluded, 757 (9.7%) reported asthma, 168 (2.2%) reported COPD, and 46 (0.6%) reported asthma and COPD at baseline.
Baseline questionnaire and exposure
Baseline data were elicited with questions regarding demographic characteristics, physical health, psychological health, psychosocial factors, socioeconomic factors, and lifestyle and social factors. These questions were included in the 2006 survey, as reported previously [24].
Exposure
Potential factors of importance for the risk of developing troublesome LBP were self-reported asthma and COPD. The questions to measure this were: (1) Do you suffer from asthma? Answered by no/yes. (2) Do you suffer from chronic obstructive pulmonary disease? Answered by no/yes.
Potential confounders
Potential confounders were chosen from the baseline questionnaire guided by knowledge from prior research and by clinical considerations [7, 26]. Potential confounders in the present study were: age (continuous and dichotomized into < 22, 23–31, 32–41 and > 41); sex (men/women); weight (kg); height (cm); smoking habits (yes, daily); alcohol consumption (some time during a period of 12 months); neck/shoulder/arm pain the previous six months (yes; more than two days); socioeconomic class (unskilled and semiskilled workers, skilled workers, assistant non-manual employee, intermediate non-manual employees, employed/self-employed/professional); main physical workload in the past 12 months (sedentary, light, moderately heavy, heavy); time spent on household work per day (yes > 10 h); economic stress based on the question “Did it happen that during the past 12 months you ran out of salary/money and had to borrow from relatives or friends in order to pay for food or rent?” (yes); experience of stress (yes, once/month or more); country of birth (Sweden/elsewhere); and leisure physical activity level (sedentary < 2 h per week/active ≥ 2 h per week); and sleep (h).
Outcome
The outcome was having experienced at least one period of troublesome LBP during the past 6 months, asked for with questions in the 2010 follow-up survey. Participants who answered “yes” to both of the following questions were defined as having had experienced troublesome LBP: “During the past six months, have you felt pain (at least a couple of days per week) in your lower back? If so, have these restricted your work capacity or hindered you in daily activities to some degree or to a high degree?”.
Statistical analysis
For the analyses of associations between the prognostic factors and the outcome, binomial regression models were used. Results are presented as risk ratios (RR), along with 95% confidence intervals (95% CI).
Potential confounding factors were investigated and added one at a time to the crude regression model. As described by Rothman and Greenland [27], a factor that changed the crude RR by 10% or more was considered a confounder and was entered into the final model.
Results
Our study population reporting that they had had LBP no or a few days the last 6 months at baseline (n = 17,177) was derived from a dataset from the SPHC 2006/2010 (n = 25,167). Among people reporting asthma in 2006 (n = 1139), 35 persons (3%) reported troublesome LBP in 2010 and among persons reporting COPD at baseline 2006 (n = 162), 11 persons (7%) reported troublesome LBP in 2010. For those suffering from both asthma and COPD at baseline (n = 37), 5 (27%) reported troublesome LBP in 2010. Fifty-four percent (n = 9224) of the cohort were women, and 45% of all (n = 7792) were aged > 41 years. Mean age of the cohort was 49 (SD 16) years and for those suffering from asthma 45 (SD16) years or for COPD 46 (SD10) years. The demographics of the study population stratified by those who suffered from asthma and COPD at baseline are presented in Table 1.
After adjusting for confounding, suffering from asthma and/or COPD at baseline was associated with reporting troublesome LBP experienced during the 6 months prior to follow-up in 2010 (Table 2). Age emerged as a confounder for the analyses of asthma and COPD, respectively. For those suffering from both asthma and COPD, age, sleep, and neck/shoulder/arm pain were confounders and included in the adjusted analysis. Adjusted results indicate that those suffering from asthma at baseline have an increased risk to experience troublesome LBP at follow-up RR 1.29 (95% CI 0.92–1.81) as do those suffering from COPD with a risk of RR 2.0 (95% CI 1.13–3.56). If they suffer from asthma and COPD, the risk was RR 3.55 (95% CI 1.58–7.98).
Discussion
Our aim was to study whether asthma or/and COPD are risk factors for reporting at least one episode of troublesome LBP 4 years later among people reporting no or only a few days of LBP during the last 6 months at baseline. Our findings show that those suffering from asthma or COPD at baseline had a higher risk to experience troublesome LBP at follow-up 4 years later. The risk of developing troublesome LBP in those with both asthma and COPD was high, but the number of exposed cases was low, despite the large study population. Our findings concur with a recent review concluding a significant correlation between the presence of LBP and RD such as dyspnea, asthma, different forms of allergy, and respiratory infections [12]. Contradictory to our findings, Beeckmans et al. [12] did not find an association between LBP and COPD, while our findings indicate a risk of developing troublesome LBP among those suffering from COPD. COPD is a disorder that is highly debilitating, and asthma is previously reported to have an impact on health-related quality of life [19, 21, 28]. The findings from Beeckmans et al. [12] are however based on mostly cross-sectional studies, while our results followed a population cohort prospectively.
To the best of our knowledge, and aligning with our study findings, there are only two previous studies investigating the association between LBP and RD over time [29]. Smith et al. [29] investigated women in a retrospective design and reported that those with respiratory problems developed LBP over time and vice versa. The cohort was also investigated for incontinence and gastrointestinal problems making the perspective of comorbidity even wider and suggesting a possible common basis for several coexisting dysfunctions [29]. In addition, Hestbaek et al. [15] in a prospective cohort found that young people reporting asthma had a greater risk of reporting persistent LBP 8 years later indicating a comorbidity (12). Apart from the aforementioned studies, the inter-relationship between LBP and RD to date has mostly been investigated in cross-sectional designs suggesting that the causal associations cannot be decided [11,12,13]. Since the proportion of older people is increasing in the population, the study of multi-morbidity of physical disorders is important, in order to decrease personal suffering and the socioeconomic costs and to increase quality of life [30].
Several neurophysiological reasons behind the inter-relationship between LBP and RD have been discussed. The diaphragm muscle is reported to play an important role in the muscular respiratory system and in addition a key role in spinal stability [17, 29, 31]. About two decades ago, Hodges and Gandevia [17] suggested that changes in the intra-abdominal pressure due to poor activation of the diaphragm was related to spinal stiffness. Bordoni et al. [19] discussed the association between a poor functioning diaphragm and COPD reporting that the diaphragm loses its function to contribute to the intra-abdominal pressure in COPD, thus impacting on the spinal stiffness or spinal posture. For those suffering from asthma, the physical mechanism seems to be similar as due to the presence of expiratory flow limitation and exercise-induced bronchoconstriction, thus making dynamic lung hyperinflation common [23].
Another possible explanation taking the multi-morbidity perspective into consideration is the association between a sedentary life style, RD and pain. People suffering from COPD reportedly have a lower daily activity level compared to healthy [32]. A recent study investigating comorbidity in chronic disorders with a sedentary behavior, in addition, reported that LBP, asthma, and chronic lung disease have been found to have an association with disability, mobility, and with chronic back and joint pain [33]. In our analyses, we tested for several confounders including a sedentary lifestyle, which, however, did not emerge as a confounding factor.
Hestbaek et al. [14] proposed in 2003 that LBP is not to be seen as an own specific entity. This implies that several disorders or symptoms co-associate and might even have the same basis of its occurrence even if the reason for this is not yet fully understood. To better understand the inter-relationship, potential modifiers or mediators are needed to clarify underlying mechanisms. In the management of people suffering from LBP, possible comorbidity and factors impacting on these such as psychosocial and socioeconomic factors therefore need to be taken into consideration when targeting preventive or rehabilitative interventions such as exercise and education for example.
Strengths and limitations
A strength of the present study is its prospective design in a general population in which the exposures asthma and COPD were measured at baseline and prior to outcome. Several potential confounders were considered: among others weight, height, smoking, sedentary life style, working condition, and physical activity level, factors that might be of importance in the association between RD and LBP. In our analyses, only age confounded the association between LBP, asthma, and COPD and was thus included in the adjusted analyses. Even so, we cannot rule out the risk of unmeasured or residual confounding such as medication, ethnicity, life style, and psychological distress, in addition to the misclassification of confounding.
A limitation to the present study is that RD were measured with a single question, which may lead to a misclassification of the exposure. However, since we have no reason to believe that a potential misclassification of the exposure is related to the outcome in a prospective cohort study, the most probable effect would be a dilution of the true association between the exposures and the outcome. Another limitation might be the low power in our analyses as we had few exposed cases at the follow-up especially for those suffering from asthma and COPD. Even so, our findings indicate a risk of people exposed to asthma and COPD to develop troublesome LBP at follow-up.
The response rate from baseline 2006 to follow-up 2010 was 73%, and therefore, there is in addition a risk of selection bias. For people reporting asthma at baseline, there was an equal part who answered and did not answer the 2010 follow-up survey. For COPD, the percentage of those who did not answer the 2010 follow-up were proportionally higher compared to those not answering meaning that there might be an underestimated risk of developing troublesome LBP in our study. The generalizability of our results extends only to cohorts considered comparable to ours.
Conclusion
Our findings based on the Stockholm Public Health Cohort indicate that suffering from asthma and/or COPD increase the risk of developing troublesome LBP in the long term, which highlights the importance to consider the overall health of people suffering from LBP, and to take the multi-morbidity perspective into consideration. Future longitudinal studies are needed to confirm our findings.
References
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2163–2196. https://doi.org/10.1016/S0140-6736(12)61729-2
Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R (2012) A systematic review of the global prevalence of low back pain. Arthritis Rheum 64(6):2028–2037. https://doi.org/10.1002/art.34347
Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 (2016). Lancet 388 (10053):1545–1602. 10.1016/S0140–6736(16)31678–6
Hestbaek L, Leboeuf-Yde C, Manniche C (2003) Is low back pain part of a general health pattern or is it a separate and distinctive entity? A critical literature review of comorbidity with low back pain. J Manipulative Physiol Ther 26(4):243–252
Deyo RA, Weinstein JN (2001) Low back pain. N Engl J Med 344(5):363–370. https://doi.org/10.1056/NEJM200102013440508
Livshits G, Popham M, Malkin I, Sambrook PN, Macgregor AJ, Spector T, Williams FM (2011) Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 70(10):1740–1745. https://doi.org/10.1136/ard.2010.137836
Balague F, Mannion AF, Pellise F, Cedraschi C (2012) Non-specific low back pain. Lancet 379(9814):482–491. https://doi.org/10.1016/S0140-6736(11)60610-7
Pincus T, Kent P, Bronfort G, Loisel P, Pransky G, Hartvigsen J (2013) Twenty-five years with the biopsychosocial model of low back pain-is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine (Phila Pa 1976) 38(24):2118–2123. https://doi.org/10.1097/BRS.0b013e3182a8c5d6
Bohman T, Alfredsson L, Jensen I, Hallqvist J, Vingard E, Skillgate E (2014) Does a healthy lifestyle behaviour influence the prognosis of low back pain among men and women in a general population? A population-based cohort study. BMJ Open 4(12):e005713. https://doi.org/10.1136/bmjopen-2014-005713
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M, Lancet Low Back Pain Series Working G (2018) What low back pain is and why we need to pay attention. Lancet 391(10137):2356–2367. https://doi.org/10.1016/S0140-6736(18)30480-X
Ferreira PH, Beckenkamp P, Maher CG, Hopper JL, Ferreira ML (2013) Nature or nurture in low back pain? Results of a systematic review of studies based on twin samples. Eur J Pain 17(7):957–971. https://doi.org/10.1002/j.1532-2149.2012.00277.x
Beeckmans N, Vermeersch A, Lysens R, Van Wambeke P, Goossens N, Thys T, Brumagne S, Janssens L (2016) The presence of respiratory disorders in individuals with low back pain: A systematic review. Man Ther 26:77–86. https://doi.org/10.1016/j.math.2016.07.011
Dimitriadis A, Kirkby KJ, Nisbet A, Clark CH (2016) Current status of cranial stereotactic radiosurgery in the UK. Br J Radiol 89(1058):20150452. https://doi.org/10.1259/bjr.20150452
Hestbaek L, Leboeuf-Yde C, Engberg M, Lauritzen T, Bruun NH, Manniche C (2003) The course of low back pain in a general population. Results from a 5-year prospective study. J Manipulative Physiol Ther 26 (4):213–219.
Hestbaek L, Leboeuf-Yde C, Kyvik KO (2006) Is comorbidity in adolescence a predictor for adult low back pain? A prospective study of a young population. BMC Musculoskelet Disord 7:29. https://doi.org/10.1186/1471-2474-7-29
Hestbaek L, Leboeuf-Yde C, Kyvik KO, Vach W, Russell MB, Skadhauge L, Svendsen A, Manniche C (2004) Comorbidity with low back pain: a cross-sectional population-based survey of 12- to 22-year-olds. Spine (Phila Pa 1976) 29(13):1483–1491 (discussion 1492)
Hodges PW (1985) Gandevia SC (2000) Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J Appl Physiol 89(3):967–976
Hodges P, Kaigle Holm A, Holm S, Ekstrom L, Cresswell A, Hansson T, Thorstensson A (2003) Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine 28(23):2594–2601. https://doi.org/10.1097/01.BRS.0000096676.14323.25
Bordoni B, Marelli F, Morabito B, Sacconi B, Caiazzo P, Castagna R (2018) Low back pain and gastroesophageal reflux in patients with COPD: the disease in the breath. Int J Chron Obstruct Pulmon Dis 13:325–334. https://doi.org/10.2147/COPD.S150401
Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR (2017) Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res 18(1):31. https://doi.org/10.1186/s12931-017-0512-2
Chen YW, Camp PG, Coxson HO, Road JD, Guenette JA, Hunt MA, Reid WD (2017) Comorbidities That Cause Pain and the Contributors to Pain in Individuals With Chronic Obstructive Pulmonary Disease. Arch Phys Med Rehabil 98(8):1535–1543. https://doi.org/10.1016/j.apmr.2016.10.016
Janssens L, Brumagne S, McConnell AK, Claeys K, Pijnenburg M, Burtin C, Janssens W, Decramer M, Troosters T (2013) Proprioceptive changes impair balance control in individuals with chronic obstructive pulmonary disease. PLoS ONE 8(3):e57949. https://doi.org/10.1371/journal.pone.0057949
Shei RJ, Paris HL, Wilhite DP, Chapman RF, Mickleborough TD (2016) The role of inspiratory muscle training in the management of asthma and exercise-induced bronchoconstriction. Phys Sportsmed 44(4):327–334. https://doi.org/10.1080/00913847.2016.1176546
Svensson AC, Fredlund P, Laflamme L, Hallqvist J, Alfredsson L, Ekbom A, Feychting M, Forsberg B, Pedersen NL, Vagero D, Magnusson C (2012) Cohort Profile: The Stockholm Public Health Cohort. Int J Epidemiol. 10.1093/ije/dys126
Svensson AC, Fredlund P, Laflamme L, Hallqvist J, Alfredsson L, Ekbom A, Feychting M, Forsberg B, Pedersen NL, Vagero D, Magnusson C (2013) Cohort profile: The Stockholm Public Health Cohort. Int J Epidemiol 42(5):1263–1272. https://doi.org/10.1093/ije/dys126
Hayden JA, Dunn KM, van der Windt DA, Shaw WS (2010) What is the prognosis of back pain? Best Pract Res Clin Rheumatol 24(2):167–179. https://doi.org/10.1016/j.berh.2009.12.005
Rothman KJ, Greenland S (2008) Introduction to stratified analysis. In: Modern epidemiology, 3rd edn. Wolters Kluwer Health/Lippincott Williams & Williams, Philadelphia, Philadelphia
Hernandez G, Dima AL, Pont A, Garin O, Marti-Pastor M, Alonso J, Van Ganse E, Laforest L, de Bruin M, Mayoral K, Ferrer M (2018) Impact of asthma on women and men: comparison with the general population using the EQ-5D-5L questionnaire. PLoS ONE 13(8):e0202624. https://doi.org/10.1371/journal.pone.0202624
Smith MD, Russell A, Hodges PW (2014) The relationship between incontinence, breathing disorders, gastrointestinal symptoms, and back pain in women: a longitudinal cohort study. Clin J Pain 30(2):162–167. https://doi.org/10.1097/AJP.0b013e31828b10fe
Picco L, Achilla E, Abdin E, Chong SA, Vaingankar JA, McCrone P, Chua HC, Heng D, Magadi H, Ng LL, Prince M, Subramaniam M (2016) Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res 16:173. https://doi.org/10.1186/s12913-016-1421-7
Hodges PW, Gandevia SC (1985) Richardson CA (1997) Contractions of specific abdominal muscles in postural tasks are affected by respiratory maneuvers. J Appl Physiol 83(3):753–760. https://doi.org/10.1152/jappl.1997.83.3.753
Vorrink SN, Kort HS, Troosters T, Lammers JW (2011) Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res 12:33. https://doi.org/10.1186/1465-9921-12-33
Vancampfort D, Stubbs B, Koyanagi A (2017) Physical chronic conditions, multimorbidity and sedentary behavior amongst middle-aged and older adults in six low- and middle-income countries. Int J Behav Nutr Phys Act 14(1):147. https://doi.org/10.1186/s12966-017-0602-z
Acknowledgements
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Primary data are available for journal review if requested.
Conflict of interest
None of the authors has any potential conflict of interest.
Ethical approval
This study was approved by Stockholm’s Regional Committee for Medical Research Ethics and conformed to the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rasmussen-Barr, E., Magnusson, C., Nordin, M. et al. Are respiratory disorders risk factors for troublesome low-back pain? A study of a general population cohort in Sweden. Eur Spine J 28, 2502–2509 (2019). https://doi.org/10.1007/s00586-019-06071-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06071-5